Phospho-EGF Receptor (Thr669) Antibody #3056
- WB
To Purchase # 3056
| Cat. # | Size | Qty. | Price |
|---|---|---|---|
| 3056S | 100 µl | $357 |
Product Specifications
| REACTIVITY | H R |
| SENSITIVITY | Endogenous |
| MW (kDa) | 175 |
| SOURCE | Rabbit |
Application Key:
- WB-Western Blotting
Species Cross-Reactivity Key:
- H-Human
- R-Rat
- Related Products
Product Information
Product Usage Information
| Application | Dilution |
|---|---|
| Western Blotting | 1:1000 |
Storage
Protocol
Specificity / Sensitivity
Species Reactivity:
The antigen sequence used to produce this antibody shares 100% sequence homology with the species listed here, but reactivity has not been tested or confirmed to work by CST. Use of this product with these species is not covered under our Product Performance Guarantee.
Species predicted to react based on 100% sequence homology:
Source / Purification
Background
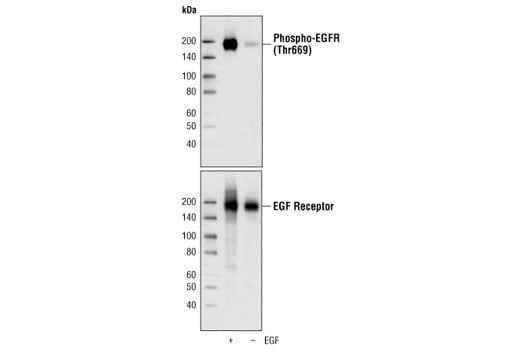
Thr669 is a major phosphorylation site on EGF receptor after EGF stimulation. It is phosphorylated by p38 MAP kinase (11). Phosphorylation of the EGF receptor at Thr669 may be involved in regulation of ligand induced receptor internalization by interacting with downstream specific EGF receptor tyrosine kinase substrate(s) (11).
- Hackel, P.O. et al. (1999) Curr Opin Cell Biol 11, 184-9.
- Zwick, E. et al. (1999) Trends Pharmacol Sci 20, 408-12.
- Cooper, J.A. and Howell, B. (1993) Cell 73, 1051-4.
- Hubbard, S.R. et al. (1994) Nature 372, 746-54.
- Biscardi, J.S. et al. (1999) J Biol Chem 274, 8335-43.
- Emlet, D.R. et al. (1997) J Biol Chem 272, 4079-86.
- Levkowitz, G. et al. (1999) Mol Cell 4, 1029-40.
- Ettenberg, S.A. et al. (1999) Oncogene 18, 1855-66.
- Rojas, M. et al. (1996) J Biol Chem 271, 27456-61.
- Feinmesser, R.L. et al. (1999) J Biol Chem 274, 16168-73.
- Winograd-Katz, S.E. and Levitzki, A. (2006) Oncogene 25, 7381-7390.
Alternate Names
EGF; EGFR; epidermal growth factor receptor; Her1
Pathways
Explore pathways related to this product.
Limited Uses
Except as otherwise expressly agreed in a writing signed by a legally authorized representative of CST, the following terms apply to Products provided by CST, its affiliates or its distributors. Any Customer's terms and conditions that are in addition to, or different from, those contained herein, unless separately accepted in writing by a legally authorized representative of CST, are rejected and are of no force or effect.
Products are labeled with For Research Use Only or a similar labeling statement and have not been approved, cleared, or licensed by the FDA or other regulatory foreign or domestic entity, for any purpose. Customer shall not use any Product for any diagnostic or therapeutic purpose, or otherwise in any manner that conflicts with its labeling statement. Products sold or licensed by CST are provided for Customer as the end-user and solely for research and development uses. Any use of Product for diagnostic, prophylactic or therapeutic purposes, or any purchase of Product for resale (alone or as a component) or other commercial purpose, requires a separate license from CST. Customer shall (a) not sell, license, loan, donate or otherwise transfer or make available any Product to any third party, whether alone or in combination with other materials, or use the Products to manufacture any commercial products, (b) not copy, modify, reverse engineer, decompile, disassemble or otherwise attempt to discover the underlying structure or technology of the Products, or use the Products for the purpose of developing any products or services that would compete with CST products or services, (c) not alter or remove from the Products any trademarks, trade names, logos, patent or copyright notices or markings, (d) use the Products solely in accordance with CST Product Terms of Sale and any applicable documentation, and (e) comply with any license, terms of service or similar agreement with respect to any third party products or services used by Customer in connection with the Products.

