A binary approach is one of the best ways to evaluate antibody specificity. By testing an antibody in biologically relevant positive and negative expression systems, it is possible to confirm that it recognizes the target antigen in its native environment without cross-reacting with other biomolecules present in the sample. Binary models include endogenous cells or tissues where expression of the target protein is known or predicted to be positive (high) or negative (low), genetic knockouts, and the use of treatments to induce or inhibit expression or modification of a protein target.
For binary testing to be effective, data should always be verified using an orthogonal method, such as genetic sequencing to confirm knockout or proteomic profiling to verify expression levels. To provide utmost confidence in antibody performance, additional validation strategies should also be employed. Moreover, each model that is used for binary validation of an antibody should be tested in each application for which the antibody is intended to be used – just because an antibody is specific by western blot does not mean that it will be as specific by immunohistochemistry (IHC); this approach is illustrated in the data below.
Cells and tissues that endogenously express the target of interest are the simplest form of positive control. Used in parallel with samples derived from similar material that does not demonstrate target expression, they provide a rapid yes/no answer regarding specific antigen recognition by an antibody.
To ensure this testing strategy delivers meaningful insight, it is important to select sample material representative of a true positive or negative control. This can be achieved by mining the literature, by utilizing genomic, transcriptomic, or proteomic databases; or comparing the immunostaining produced by the test antibody against that of a fully validated antibody against a distinct antigen on the same target.
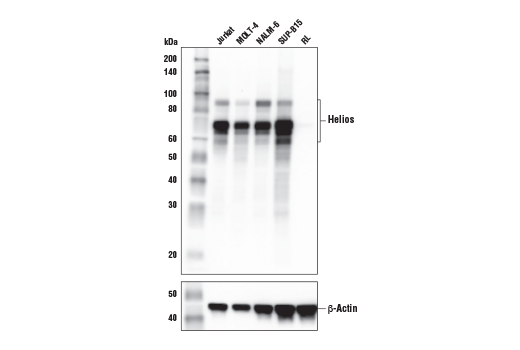
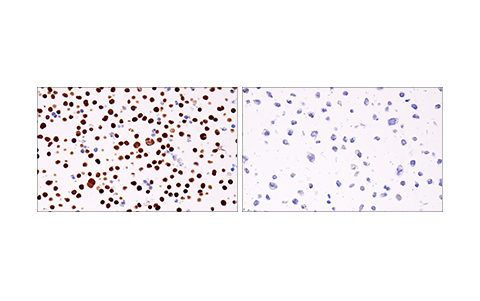
Figures 1 and 2 provide examples of antibody validation using endogenous controls, highlighting the concept that models used to validate an antibody should be tested in each intended application. Here, Figure 1 shows western blot evaluation of the Helios (E4L5U) rabbit mAb in Jurkat and RL cells, which are positive and negative for Helios expression respectively, while Figure 2 shows IHC evaluation of the antibody in the same cell models. As shown, the IHC and western blot data support each other, with loading controls ensuring that sample (analyte) quality does not lead to misinterpretation of results.
Endogenous binary models are not always an option since some proteins are ubiquitously expressed, or data to support expression may be conflicting or unreliable. In these cases, mouse knockout models are widely used controls for binary evaluation of antibody specificity. Typically provided by collaborators, mouse knockout samples comprise wild-type and knockout cells and tissues. These exhibit native expression and no expression of the target antigen respectively, and represent an ideal testing paradigm when available.
Another method to achieve genetic knockout is to use CRISPR-mediated gene disruption, a technique which has seen increased utility in recent years following its simplification into an intuitive and highly flexible system. An advantage of this approach is that it provides a positive and negative expression model in the same cell line. However, caution must be applied because disruption of the gene is not always complete, sometimes resulting in truncations or gene fragments that may yield signal. It is important that target knockdown be confirmed using an orthogonal, antibody-independent strategy. Additionally, it is important to note that CRISPR knockout in cells, while very informative about an antibody’s performance in cell-based assays, does not necessarily translate to tissue-based assays, like IHC, in which multiple cell types are present.
In addition to CRISPR-mediated knockdown, small (or short) interfering RNA (siRNA) is a synthetic RNA duplex that is commonly used to induce short-term silencing of protein coding genes. Yet another option, short or small hairpin RNA (shRNA) is typically contained within a DNA vector and introduced into cells via transfection or viral transduction. Gene silencing results from the expression of the shRNA, with the possibility of prolonged silencing if the transfected or transduced cells are selected for by leveraging the selection marker of a vector, or if there is stable integration of the shRNA into the host genome.
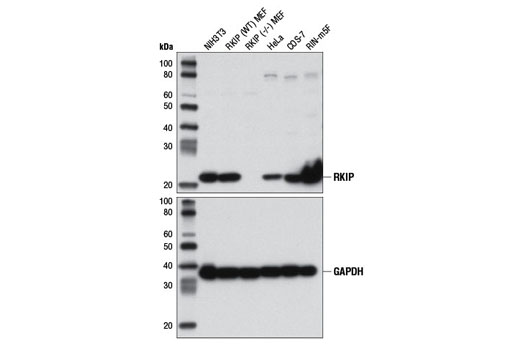
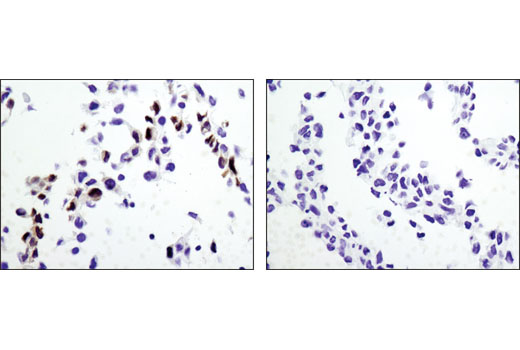
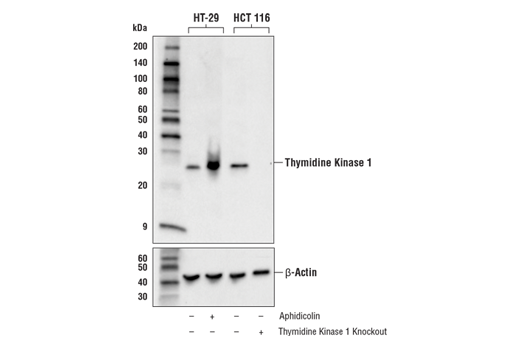
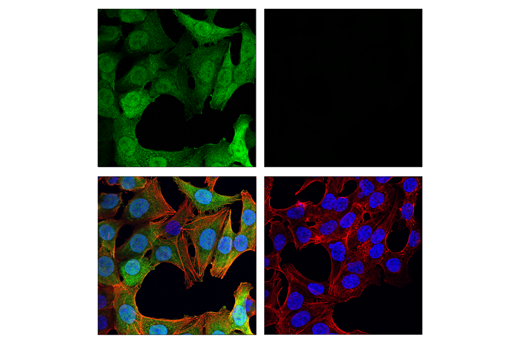
Again, it is critically important that the genetic knockout models used to validate an antibody are tested in each anticipated application independently. Figures 3 and 4 show western blot and immunohistochemistry assessment of the RKIP (D42F3) rabbit mAb in wild-type and RKIP knockout mouse embryonic fibroblast (MEF) cells, while Figures 5 and 6 illustrate western blot and immunocytochemical analysis using the Thymidine Kinase 1 (E2H7Z) rabbit mAb to probe wild type or thymidine kinase 1 knockout HCT 116 cells. As shown, the IHC and western blot data support each other.
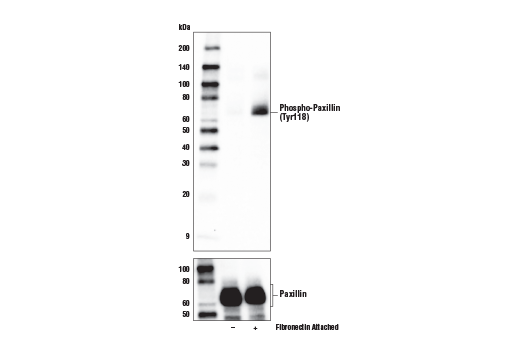
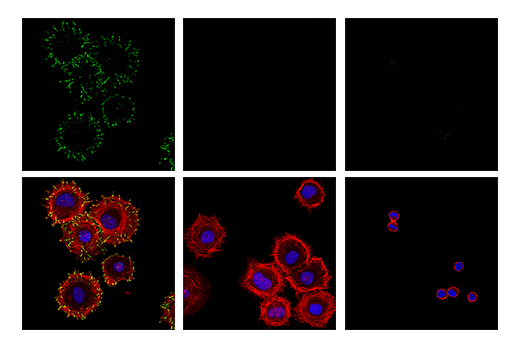
Another form of binary model involves the use of treatments (agonists or antagonists) to induce or inhibit the expression, localization, or post-translational modification (PTM) of a protein target. Where these treatments result in near-complete positive and negative signal, they can be considered binary validation. Target antigen modification is a preferred approach to validate antibodies against post-translationally modified targets such as phosphorylated or acetylated proteins.
As with any validation technique, suitable loading and expression controls are of critical importance to validation methods that are reliant on modification of the target antigen. These are used to confirm the efficacy of the treatment and to ensure that the samples being evaluated are equivalent in both quality and quantity. It is also important that the treatment is as specific to the target as possible. For example, a specific agonist or inhibitor of a protein or pathway should always be considered before using a more general treatment like phosphatases or deacetylases that nonspecifically remove PTMs from a broad range of targets.
Figures 7 and 8 show validation of a phospho-paxillin (Tyr118) antibody in HeLa cells, using cellular attachment to a fibronectin-coated surface to stimulate phosphorylation on the tyrosine residue, resulting in induced immunocytochemical labeling.
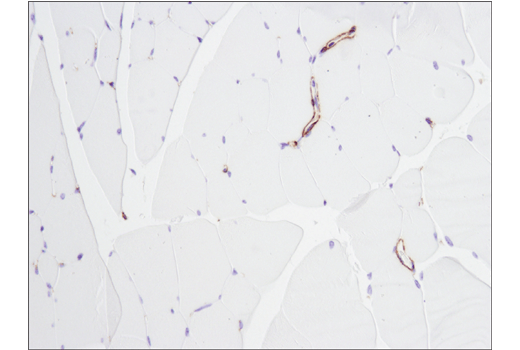
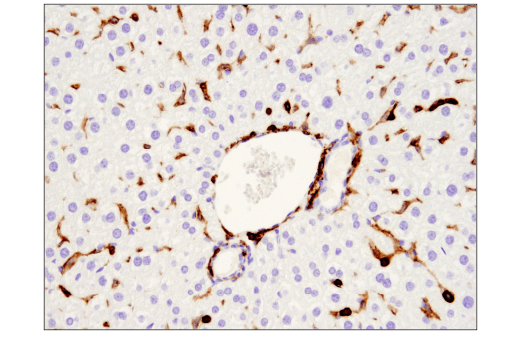
An additional binary strategy, most frequently used in IHC, involves validating an antibody in tissue samples that contain both positive and negative cells within the same section. This is illustrated in Figures 9 and 10, where the observed staining pattern is consistent with the known expression profile of the targets (ie, positive staining in the correct cells and no staining in the negative cells).
Although binary models provide valuable insight, they are not always readily available or easy to generate quickly for the purpose of validating an antibody reagent. To support binary testing data, or as an alternative means of assessing antibody performance, additional validation methods should always be employed.