Antibody Validation for Flow Cytometry
Our flow-validated products undergo rigorous testing in biologically relevant models, ensuring specificity and an optimal signal-to-noise ratio (S/N) for both conjugated and unconjugated antibodies. Cross-platform validation further confirms antibody specificity. In addition, all antibodies have been tested for optimal dilution, specificity, stability and lot-to-lot reproducibility to ensure they work the first time, every time.
Our thoroughly validated antibodies for flow cytometry make it possible to examine complex intracellular signaling cascades in cell lines, dissociated tissues, aspirates, or hematology specimens, and trust the accuracy of your results.
Validation Includes:
- Use of positive and negative cell lines
- Comparison of signal to isotype control to estimate nonspecific binding of primary antibodies
- Treatment with pathway-specific inhibitors/activators
- Treatment with blocking peptides, siRNA, and/or expression vectors
- Phosphatase treatment to confirm phospho-specificity
- Extensive quality control testing to guarantee stability over time and to eliminate lot-to-lot variability
- Optimization of protocols and determination of optimal dilutions
- Validation across multiple applications to confirm antibody specificity
The performance of our antibodies is routinely validated across multiple platforms. Below is an example, which demonstrates why such testing is necessary.
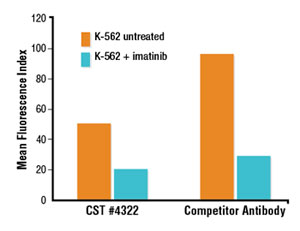
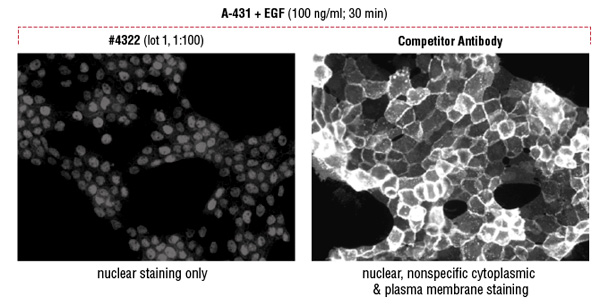
Comparative analysis in flow cytometry and immunofluorescence confirms that the CST antibody detects the target specifically. The signal correctly localizes to the nuclear compartment of the cell, although the signal is less robust.
A.
B.
Flow cytometric analysis suggests a brighter signal from a competitor’s Phospho-Stat5 (Tyr694) antibody compared to a lower fold induction with Phospho-Stat5 (Tyr694) (D47E7) XP® Rabbit mAb #4322 (A). However, immunofluorescent analysis reveals that the competitor antibody inappropriately stains the cytoplasm and plasma membrane, while #4322 demonstrates only the appropriate nuclear staining (B).