Validation for Histone Modification Antibodies
Antibodies targeted to histone modifications may bind non-specifically to similar, but off-target histone modifications. Conversely, their specific binding can be inhibited by steric hindrance from modifications on neighboring residues. Assays like ELISA, western blot, ChIP, and IF are commonly used to demonstrate antibody specificity and sensitivity, but they cannot clearly predict how an antibody will interact with nearby epitopes. As a result, they are of limited use when trying to validate an antibody to a histone modification target.
For these reasons, CST modification-specific histone antibodies are validated using a peptide array assay similar to the one described by Fuchs, S.M., et al. (1). In a single experiment, these arrays assess reactivity against known modifications across all histone proteins as well as the effects of neighboring modifications on the ability of the antibody to detect a single modification site. Therefore, the peptide array assay allows us to confirm the antibodies are performing as expected.
Array
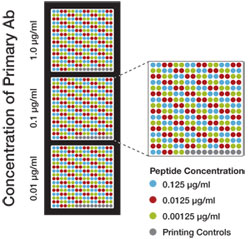
Peptides with mono-, di-, tri-methyl, acetyl-, or unmodified lysine are spotted onto nitrocellulose either alone or in combination with a known neighboring histone modification (e.g., histone H3K4Me3 and H3T3Phos), as indicated in the diagram. A similar array is used for testing methyl-arginine antibodies.
Antibody
The histone modification antibody is applied to the array at three concentrations, as indicated in the diagram. This allows us to assess antibody reactivity while ensuring that the antibody concentration is not saturating the assay.
Analysis
The arrays are washed and incubated with a fluorescently tagged secondary antibody and then read using a LI-COR Odyssey Infrared Imager.
Please click below to see examples of the analysis we perform on our histone modification antibodies.