The History of CST
1999
Founding of CST
Founded in 1999 by Michael J. Comb, PhD, a neuroscientist first at Massachusetts General Hospital and then at New England Biolabs, Cell Signaling Technology (CST) began as a small group of dedicated employees. Focused on signaling, CST was among the first to develop and commercialize antibodies to post-translational modifications (PTMs), helping to make the study of PTMs more mainstream and accessible. The first product offered was Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) Antibody #9101, which has been cited in over 8,000 publications and continues to be used to this day.
Social and Environmental Responsibility
Social and environmental responsibility has always been a core value at CST. Inspired by their father, Donald G. Comb, founder of New England Biolabs, Michael, CEO of CST, and his brother Dave Comb, the company’s first Creative Director, have been staunch environmentalists. As a global company of caring people driven by a devotion to facilitating good science, CST remains committed to doing the right thing for our customers, communities, and the planet.
The first CST company photo
2000-2004
Growth and a New Headquarters
At the start of the new millennium, Cell Signaling Technology had doubled in size, and established a new headquarters in Beverly, Massachusetts, USA. The move helped enable the growing team to focus on new products and innovations, including a motif antibody technology, which enabled all future proteomics work at CST. Around this time, CST launched PhosphoSite (now PhosphoSitePlus® PTM Database), a curated and free online database of protein phosphorylation sites.
New Sustainability Efforts
CST continued to expand its environmental sustainability efforts, including a new recycling and waste-reduction program and the formation of its first green committee. During this time, the first corporate donations committee and the summer internship program were formed.
1,151 Total Products
33 Total CST-Authored Papers
2005
LEED Certification
The company was growing in the early 2000s, exceeding 100 employees and 1,000 products. In 2005, CST moved its headquarters to a converted hotel in Danvers, Massachusetts, where it is still located today. The new building earned its LEED certification in 2007, making CST just the fourth biotech in New England to earn the certification.
Cell Signaling Technology headquarters in Danvers, MA, USA
1,478 Total Products
45 Total CST-Authored Papers
2006
Recombinant Monoclonal Antibody Technology
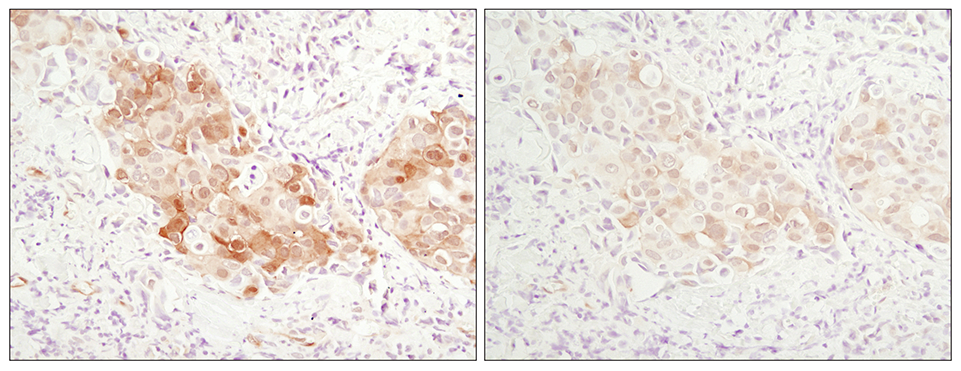
In 2006 CST developed a breakthrough recombinant technology for manufacturing monoclonal antibodies. The XMT® technology was used to develop Phospho-Akt (Ser473) (D9E) XP® Rabbit mAb #4060, the highest-cited primary antibody of all time, with over 10,000 citations.
Immunohistochemical analysis of paraffin-embedded human breast carcinoma comparing SignalStain® Antibody Diluent #8112 (left) to TBST/5% normal goat serum (right) using Phospho-Akt (Ser473) (D9E) XP Rabbit mAb #4060.
1,920 Total Products
60 Total CST-Authored Papers
2007
Discovery: The Role of ALK/ROS in Lung Cancer
One of the first and most significant breakthroughs at Cell Signaling Technology was the use of PTMScan to discover the role of ALK/ROS fusion proteins in non-small cell lung cancer. This breakthrough helped pave the way for the development of life-saving therapeutics for the 4% of lung cancer patients with this cancer type. CST scientist Klarisa Rikova was the first author of the publication, which describes how phosphotyrosine signaling helped uncover the novel fusion protein.
2.511 Total Products
80 Total CST-Authored Papers
2008-2011
Global Expansion
As CST neared its first decade in business, major growth was underway with operations expanding to Tokyo, Japan; Shanghai, China; and Leiden, Netherlands. Establishing a legal entity in Shanghai also allowed for a new collaboration with the Chinese Society for Cell Biology (CSCB).
Corporate Social Responsibility
The commitment to sustainability and corporate social responsibility was strengthened with the formal establishment of a Corporate Social Responsibility (CSR) program. Inaugural director David Comb continued the work that he and his brother Michael, CEO of CST, started many years earlier
Awards and Accolades
- Life Science Industry Award for Best Performing Antibodies 2011
- Life Science Industry Award for Best Breakthrough Products in Cancer Research 2011
5,243 Total Products
151 Total CST-Authored Papers
2012
Monoclonal Antibody Development Capabilities
Harnessing the power of proteomics, CST developed a discovery platform for antigen-specific monoclonal antibodies. Named NG-XMT™, the technology enables the isolation of monoclonal antibodies with antigen-specific activities that recapitulate or surpass those of the original affinity-purified polyclonal antibodies found in the sera of immunized rabbits and mice.
6,114 Total Products
167 Total CST-Authored Papers
2013-2016
ISO 9001 Certification
In 2013 Cell Signaling Technology completed the renovation of a second site in Beverly, Massachusetts, USA, which included energy-efficient upgrades. The following year, both of the sites in the USA received ISO 9001 certification. Looking to further reduce environmental impact, EV charging stations were installed and a high-efficiency ULT freezer program was piloted.
Scientific Reproducibility Advocacy
Roberto (Roby) Polakiewicz, Chief Scientific Officer at CST demonstrated his commitment to scientific reproducibility in the Nature article Antibodies: The solution is validation, where he advocated for greater accountability from antibody vendors in the production of high-quality antibodies.
Awards and Accolades
- CiteAb Award for Antibody Company of the Year 2014, 2016
- CiteAb Award for Researcher’s Choice 2014
- Life Science Industry Award for Best Antibodies 2014
- Boston Business Journal’s Top Charitable Companies in Massachusetts 2016
- Biocompare Antibody Market Report: Ranked #1 in Specificity, Sensitivity, Reproducibility, Technical Support, and Most Useful Website 2016
Solar panels on roof of the new Cell Signaling Technology site in Beverly, MA, USA.
8,546 Total Products
251 Total CST-Authored Papers
2017
PhosphoSitePlus: Open-Source Bioinformatics Database of PTMs
As a pioneer of antibodies used to study PTMs, CST made further contributions to the field with the implementation of PhosphoSitePlus, an improved and expanded version of the PhosphoSite database. This first-in-class bioinformatics resource helped to facilitate the annotation and study of PTMs in proteins and is publicly available for the entire community of scientists studying cellular signaling.
Anti-Counterfeiting Efforts
To combat counterfeiting attempts, the team in China introduced anti-counterfeiting measures in their region, incorporating a unique code on our packaging to assure customers that they are always getting authentic CST products.
Improved Customer Support
In Japan, a new dealer service desk was created to provide a dedicated phone number and email address to improve customer support.
Awards and Accolades
- CiteAb Award for Researcher’s Choice 2017
- CiteAb Award for PTM Antibody Company of the Year 2017
- Boston Business Journal’s Top Charitable Companies in Massachusetts 2017
- Biocompare Antibody Market Report: Ranked #1 in Specificity, Sensitivity, Reproducibility, Technical Support, and Most Useful Website 2017
9,108 Total Products
266 Total CST-Authored Papers
2018
Lifetime Achievement Award
In 2018, Cell Signaling Technology received three CiteAb awards: Antibody Company of the Year, Antibody Company Succeeding in Cancer Biology, and a Lifetime Achievement Award for CEO Dr Michael J. Comb. CST has won in at least one category nearly every year since the CiteAb awards began.
Growth and Recognition in China
To accommodate new growth, CST China moved to a new office in Shanghai, with the new facility tripling its office and warehouse footprints. ALK (D5F3®) XP® Rabbit mAb #3633 and ROS1 (D4D6®) Rabbit mAb #3287 were recommended for conventional immunohistochemical (IHC) testing in an article authored by the Chinese Society of Clinical Oncology.
Awards and Accolades
- CiteAb Award for Antibody Company of the Year 2018
- CiteAb Award for Antibody Succeeding in Cancer Biology 2018
- CiteAb Lifetime Achievement Award for CEO Dr. Michael J Comb 2018
- CiteAb Award for Chemical Probes Supplier to Watch (Highly Commended) 2018
- CiteAb Award for Antibody Company with Sustained Success in China (Highly Commended) 2018
- Life Science Industry Award for Most Responsive Customer Service 2018
- Boston Business Journal’s Top Charitable Companies in Massachusetts 2018
Michael Comb, CEO of CST, and Roberto Polakiewicz, CSO of CST, with Andrew Chalmers, CEO of CiteAb, holding the awards won in 2018.
9,681 Total Products
277 Total CST-Authored Papers
2019
A Novel Method of Chromatin Profiling
In its 20th year, CST was the first company to bring a commercial CUT&RUN kit to the market, as well as a suite of CUT&RUN-validated antibodies. Cleavage Under Targets & Release Using Nuclease (CUT&RUN) is a novel method of chromatin profiling that is faster and easier than ChIP/ChIP-seq.
Awards and Accolades
- CiteAb Award for Life Science Reagent Supplier of the Year 2019
- CiteAb Award for Antibody Validation Initiative 2019
- Biocompare Antibody Market Report: Best Antibody Reproducibility 2019
- Bioz Rapid Star Award for Fastest Growing Antibody Supplier 2019
- Boston Business Journal’s Top Charitable Companies in Massachusetts 2019
10,338 Total Products
291 Total CST-Authored Papers
2020
COVID Response
With all of the challenges of 2020, Cell Signaling Technology worked to keep its employees, their families, and the community safe. Non-critical operations were ramped down, while work continued on COVID-related development projects, such as SARS-CoV-2 Spike Protein Serological IgG ELISA Kit #20154, a new kit for the qualitative detection of human IgG antibodies against the SARS-CoV-2 spike protein in serum or plasma samples. There was no disruption in shipping or the support of customers, who continued to perform essential research to understand the virus.
Rising Black Scientists Awards
In further support of the scientific community, CST, in association with Cell Press, and the Elsevier Foundation, created the Rising Black Scientists Awards. The annual essay competition was designed to help improve representation and provide funding to support the professional development of early career researchers in the fields of life, health, earth, environmental, data, and physical sciences.
Antibody Supplier of the Decade
Having celebrated twenty years in business in 2019, CST was awarded Antibody Supplier of the Decade in the 2020 CiteAb awards. CST earned this honor as the company with the largest market share of antibody citations for its products from 2010-2019.
Awards and Accolades
- CiteAb Award for Antibody Supplier of the Decade 2020
- CiteAb Award for Antibody Supplier Succeeding in Alzheimer’s Research 2020
- CiteAb Award for ELISA Kit Supplier to Watch in 2020 (Highly Commended)
- CiteAb Top 100 Antibodies of 2019 (Announced in 2020)
- 6 of the 10 most cited antibodies were from CST
- Bioz Rising Star Award for New Up-and-Coming ChIP Reagent Supplier 2020
- Boston Business Journal’s Top Charitable Companies in Massachusetts 2020
10,979 Total Products
308 Total CST-Authored Papers
2021
Awards and Accolades
- CiteAb Award for Antibody supplier succeeding in cardiovascular research (Highly Commended) 2021
- CiteAb Top 100 Antibodies of 2020 (Announced in 2021)
- 7 of the 10 most cited antibodies were from CST
- 37 of the top 100 most cited antibodies were from CST (more than any other supplier)
- Bioz All Star Award for highest average Bioz Stars score for epigenetics products 2021
- Boston Business Journal’s Top Charitable Companies in Massachusetts 2021
11,895 Total Products
318 Total CST-Authored Papers
2022
First Life Science Member of 1% for the Planet
In 2022, CST became the first life science company to join 1% for the Planet and its global network dedicated to tackling our planet’s most pressing environmental issues. This means a commitment of 1% of the company’s total annual revenue is donated directly to nonprofit organizations working to help preserve the Earth’s biodiversity, mitigate climate change, and protect our planet for future generations—and future scientists who will follow in our footsteps.
The Kinase Library: A Revolutionary Kinase Prediction Tool
CST worked with Lewis Cantley, PhD, Michael Yaffe, PhD, and Benjamin Turk, PhD to develop and host the Kinase Library on the PhosphoSitePlus database, a revolutionary tool that uses a powerful algorithm to predict the kinase responsible for modifying a particular protein substrate.
Awards and Accolades
- CiteAb Award for Recombinant Antibody Supplier of the Year 2022
- CiteAb Award for Supplier Succeeding in Cancer Research 2022
- CiteAb Award for Antibody Supplier of the Year (Highly Commended) 2022
- CiteAb Top 100 Antibodies of 2021 (Announced in 2022)
- 8 of the top 12 most cited antibodies were from CST
- Over ⅓ of the 100 most cited antibodies were from CST (more than any other supplier)
- Bioz Rapid Top Star Award for most citations of epigenetics products 2022
- Bioz Rapid Star Award for greatest increase in number of citations of antibody products 2022
- Boston Business Journal’s Top Charitable Companies in Massachusetts 2022
13,079 Total Products
327 Total CST-Authored Papers
2023
A CAR-T Research Breakthrough
CST was recognized in three categories in the 2023 CiteAb Awards, including the Innovation Award for its new CST® anti-CAR linker antibodies, which help streamline the development and optimization of chimeric antigen receptors (CAR) for T-cell therapy research.
Parkinson’s Research Collaboration
As part of a new partnership with The Michael J. Fox Foundation for Parkinson's Research (MJFF), scientists from CST and MJFF are collaborating to identify and develop high-quality monoclonal antibodies for promising Parkinson’s disease (PD) targets. CST also partnered with several non-profit organizations focused on laboratory sustainability, networking, and STEM programs, continuing its commitment to achieving net-zero emissions by 2029.
Awards and Accolades
- Boston Business Journal’s Top Charitable Companies in Massachusetts 2023
- CiteAb Award for Innovation 2023
- CiteAb Award for Post Translational Modification Antibody Supplier of the Year 2023
- CiteAb Top 100 Antibodies of 2022 (Announced in 2023)
- 6 of the top 10 most cited antibodies were from CST
- Over ⅓ of the 100 most cited antibodies were from CST
- Ecovadis Sustainability Bronze Medal 2023
- Top Workplaces USA Award 2023
14,412 Total Products
345 Total CST-Authored Papers
2024
Commitment to Innovation, Quality, and Reproducibility
CST was honored with three 2024 CiteAb awards. The Award for Significant Individual Impact was awarded to Chief Scientific Officer Roberto (Roby) Polakiewicz, PhD for his commitment to high-quality antibody manufacturing, including the development of novel antibody methods and steadfast advocacy for scientific reproducibility standards for antibody manufacturers.
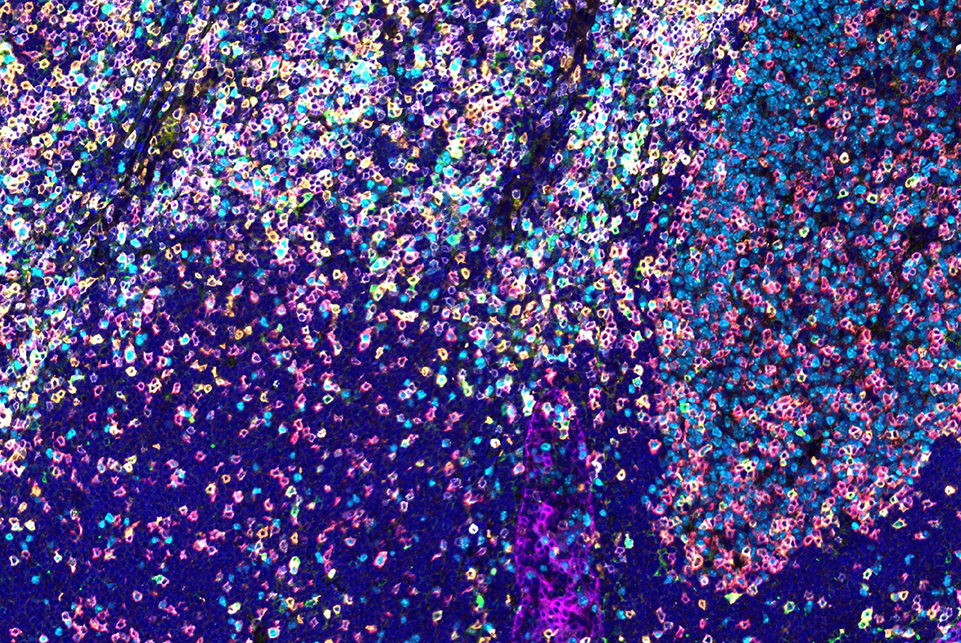
Spatial Biology Innovation
CST was also Highly Commended for the CST®SignalStar™ Multiplex Immunohistochemistry (mIHC) assay in CiteAb’s Innovation Award category. The solution enables the quantification of multiple biomarkers simultaneously using customizable, flexible, and high-validated antibody panels.
Continued Chromatin Profiling Expansion
CST further expanded its chromatin profiling with the addition of a kit and validated antibodies for Cleavage Under Targets & Tagmentation (CUT&Tag). CUT&Tag saves even more time by integrating the majority of DNA library preparation within the CUT&Tag experiment itself.
Awards and Accolades
- CiteAb Award for Award for Significant Individual Impact Roberto (Roby) Polakiewicz, PhD, CSO of CST 2024
- CiteAb Award for Antibody Supplier Succeeding in South Korea 2024
- CiteAb Award for Innovation (Highly Commended) 2024
- CiteAb Top 100 Antibodies of 2023 (Announced in 2024)
- The top 3 most cited antibodies were from CST
- 9 of the top 10 most cited antibodies were from CST
- Over 1/3 of the top 100 most cited antibodies were from CST
- Bioz All Star Award for highest average Bioz Stars score for epigenetics products 2024
- Ecovadis Sustainability Bronze Medal 2024
SignalStar™ multiplex immunohistochemical analysis of paraffin-embedded human tonsil using ICOS (D1K2T™) & CO-0027-647 SignalStar™ Oligo-Antibody Pair #24147
15,327 Total Products
356 Total CST-Authored Papers