SignalStar™ Spatial Validated Antibodies
SignalStar Multiplex IHC technology labels multiple cellular proteins simultaneously in FFPE tissue samples using specific primary antibodies and fluorescent detection reagents. It provides high accuracy and reliability in visualizing and analyzing protein expression in tissue samples for spatial biology research.
CST validates all our antibodies in each application independently, and SignalStar mIHC antibodies are validated specifically for use with SignalStar technology. That means we guarantee SignalStar antibodies will work as expected in your assay every time.
Antibody Validation Process
- All SignalStar antibodies must pass a rigorous IHC-P validation process before SignalStar validation is performed.
- SignalStar technology results are directly compared to results from traditional DAB chromogenic labeling of serial sections to confirm specificity.
- SignalStar oligo-conjugated antibodies are optimized across numerous multiplexed panel variations, in all available fluorescent channels and imaging rounds.
- SignalStar assays are validated across unique replicates, fluorescent imaging instrumentation, days, and automated versus manual protocols.
- Chromogenic staining using SignalStar oligo-conjugated antibodies is used to confirm antigen specificity that is maintained throughout the conjugation process.
- Antibodies are tested on positive control tissues known to express biomarkers of interest.
- Subcellular localization and co-localization of biomarkers are considered.
- SignalStar oligo-conjugated antibody performance is assessed in relevant models of cancer, with human cancer tissue arrays used to demonstrate antibody performance over a broad spectrum of tissue types.
- Thorough lot testing ensures the reproducibility necessary for accurate SignalStar results with CST® spatial validated antibodies. Dilutions and protocols are predetermined and specified.
Chromogenic Comparison
Percent Positive Cells/Field of View | ||||||
| 4-plex Duplicates | DAB | ||||
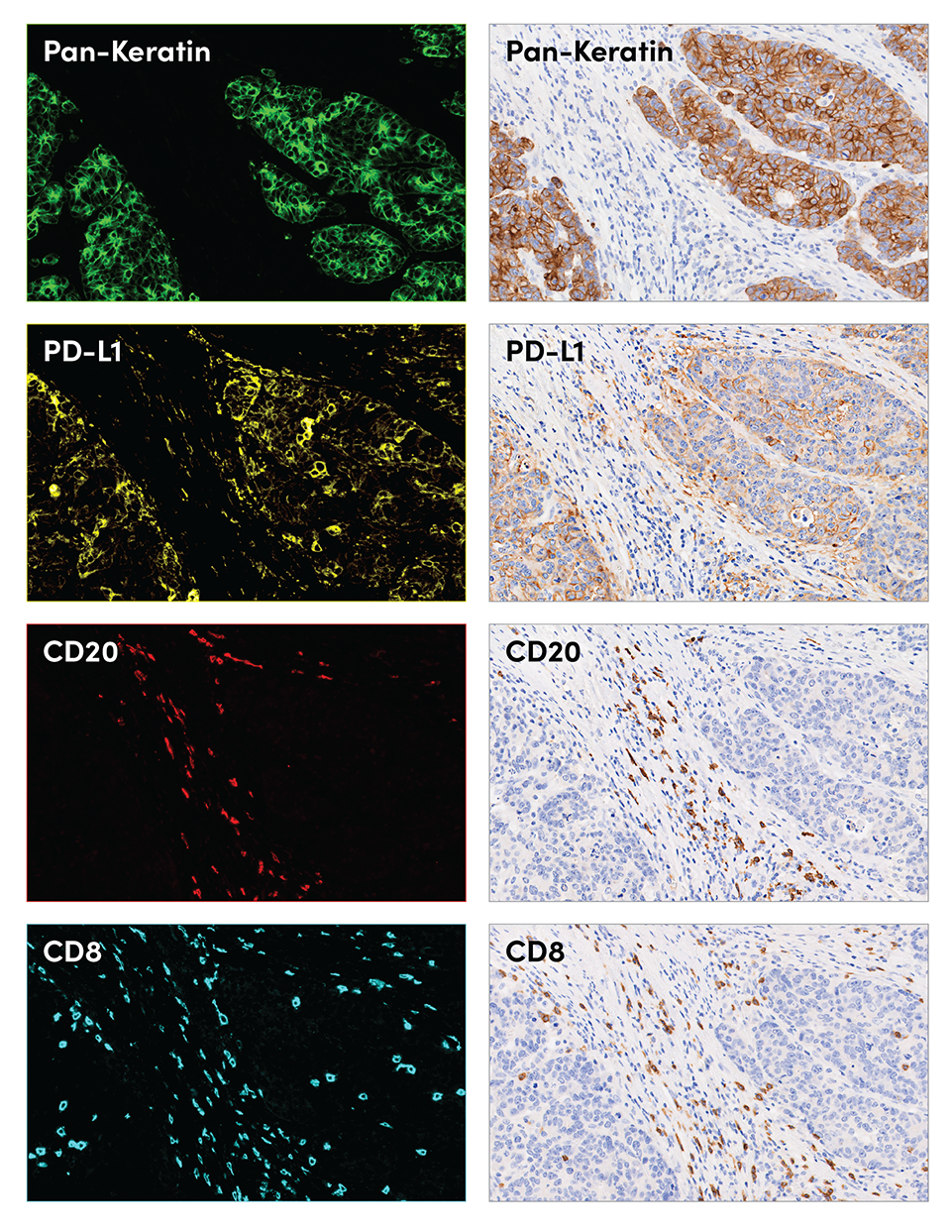
Pan-Keratin | 49.0% | 54.0% | 59.0% | |||
PD-L1 | 19.3% | 23.4% | 25.6% | |||
CD20 | 1.3% | 1.5% | 2.4% | |||
CD8 | 8.7% | 10.7% | 8.1% | |||
SignalStar multiplex immunohistochemical analysis of paraffin-embedded human colorectal adenocarcinoma using Pan-Keratin (C11) & CO-0003-488 SignalStar™ Oligo-Antibody Pair #63566 (green), PD-L1 (E1L3N®) & CO-0005-594 SignalStar™ Oligo-Antibody Pair #28249 (yellow), CD20 (E7B7T) & CO-0011-647 SignalStar™ Oligo-Antibody Pair #36775 (red), and CD8α (D8A8Y) & CO-0004-750 SignalStar™ Oligo-Antibody Pair #62750 (cyan). Representative individual and 4-plex images are included. All fluorophores were assigned a pseudocolor, as indicated. Staining was performed on the BOND RX autostainer by Leica Biosystems. Percent positive cells were quantified in matched regions of interest from serial sections for the SignalStar assay (n=2) compared to the chromogenic.
Conjugate Optimization
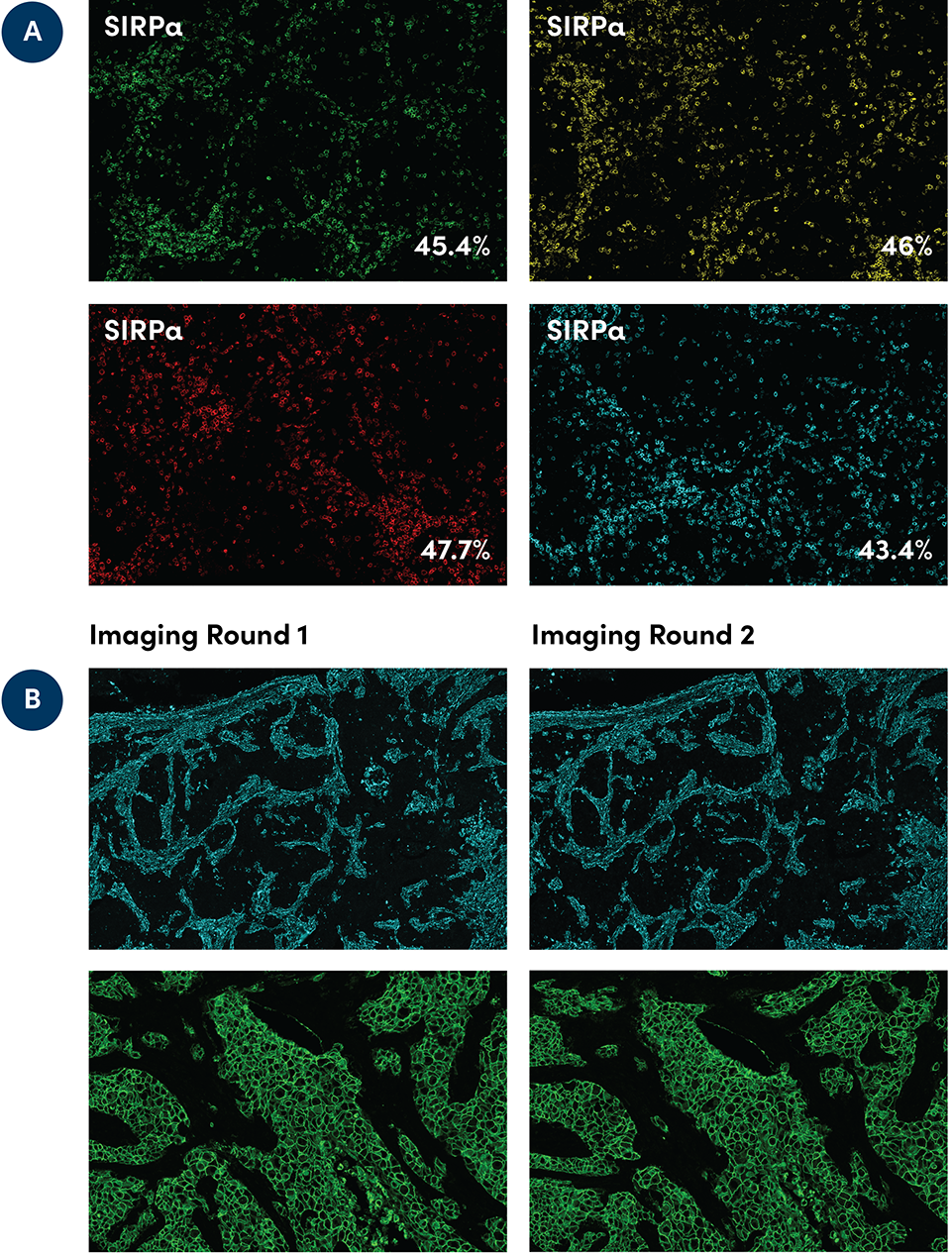
A) SignalStar immunohistochemical analysis of paraffin-embedded human squamous cell lung carcinoma using SIRPα (D6I3M) (SignalStar Conjugate 0034) in the 488 (green), 594 (yellow), 647 (red) & 750 (cyan) fluorescent channels. Percentages of positive cells in the representative ROI were quantified. B) SignalStar immunohistochemical analysis in serial sections of paraffin-embedded human infiltrating ductal carcinoma of the breast, the first imaging round (left) compared to the second imaging round (right), using Vimentin (D21H3) CO-0012-750 SignalStar™ Oligo-Antibody Pair #61002 (top panel, cyan) or HER2/ErbB2 (D8F12) & CO-0019-488 SignalStar™ Oligo-Antibody Pair #98860 (bottom panel, green). All fluorophores have been assigned a pseudocolor, as indicated. Staining was performed on the BOND RX autostainer by Leica Biosystems.
Protocol Validation
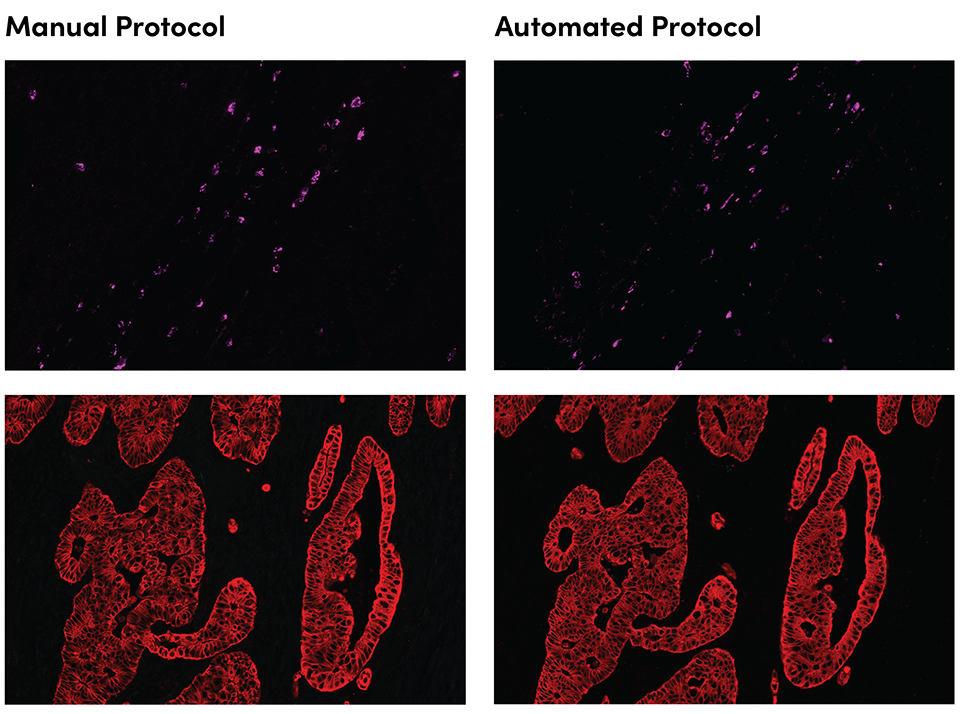
SignalStar immunohistochemical analysis in serial sections of paraffin-embedded human colorectal adenocarcinoma, with staining performed using the manual protocol (left) compared to the automated Bond RX protocol (right), using Granzyme B (D6E9W) & CO-0009-647 SignalStar™ Oligo-Antibody Pair #37369 (magenta) or Pan-Keratin (C11) & CO-0003-647 SignalStar™ Oligo-Antibody Pair #16373 (red). All fluorophores have been assigned a pseudocolor, as indicated.
Technical Support
Technical Support page to search for SignalStar-related troubleshooting information and answers to technical questions.
Cell Signaling Technology, CST, and SignalStar are trademarks of Cell Signaling Technology, Inc. All other trademarks are the property of their respective owners. Visit cellsignal.com/trademarks for more information.
U.S. Patent No. 10,781,477, foreign equivalents, and child patents deriving therefrom.