BH1-4 Protein Domains
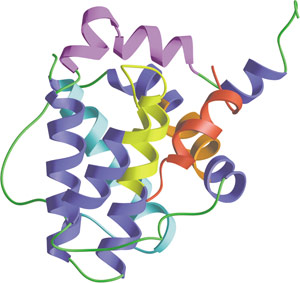
The BH1-4 domain of Bcl-xL bound to Bak peptide (red).
Domain Binding and Function
Programmed cell death, or apoptosis, is controlled by a number of proteins, among which are members of the widely expressed Bcl-2 family. Bcl-2 Homology (BH) domains are found in all proteins belonging to the Bcl-2 family. There are four distinct BH domains - BH1, BH2, BH3, and BH4. Anti-apoptotic Bcl-2 family proteins (including Bcl-2, Bcl-xL and Bcl-xW) share BH1 and BH2 domains and in some cases, a BH4 domain. Most pro-apoptotic family members, such as Bax and Bak, contain a BH3 domain, which may also be present in some anti-apoptotic proteins as well (i.e. Bcl-2 and Bcl-xL). BH domain proteins control mitochondrial-induced apoptosis by either promoting or preventing cytochrome c release into the cytosol and the subsequent regulation of caspase-9 activity. The BH3 domain promotes dimerization of Bcl-2 family members. Homodimerization of Bcl-2 involves a head-to-tail interaction in which the N-terminal region, containing the BH4 domain, interacts with the more distal region of Bcl-2 where BH1, BH2 and BH3 are located. Conversely, Bcl-2/Bax heterodimerization involves a tail-to-tail interaction that requires the BH1, BH2 and BH3 domains of Bcl-2 and a central region of Bax that contains a BH3 domain.
Structure Reference
- Sattler, M. et al. (1997) Science 275(5302), 983–986.
Examples of Domain Proteins
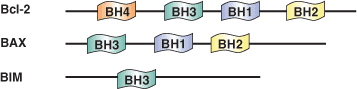
Binding Examples
BH1-4 Domain Proteins | Binding Partners |
Bcl-2, Bcl-xL (BH1, BH2, BH3) | Bax, Bad (BH3) |
Bcl-2 (BH4) | Bcl-2 (BH1, BH2, BH3) |
Mcl-1 | Bim, Noxa, PUMA (BH3) |

