BRCT Protein Domain
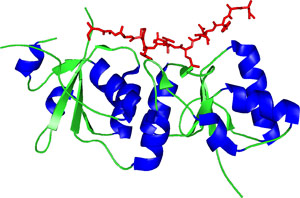
The C-terminal BRCT domain of XRCC1.
Domain Binding and Function
The BRCA1 C-Terminal domain (BRCT) was first identified by sequence analysis of the C-terminus of the BRCA1 gene. This region contains a pair of repeats that are altered (truncation or deletion) in cancers, which indicates that this region is essential for BRCA1tumor suppressor function. While single BRCT repeats have been found (e.g. XRCC1 and DNA ligase III), most identified BRCT proteins have multiple BRCT repeats. BRCT containing proteins direct protein-protein interactions during DNA repair, DNA damage response and cell cycle regulation. In a number of cases, tandem BRCT repeats constitute a phosphopeptide binding domain with ligand specificity encoded in residues C-terminal to the critical phospho-serine. Phosphopeptide binding occurs in a groove that involves both the N- and C-terminal repeats and exhibits specificity for phospho-serine containing peptides.
Structure
The BRCT domain consists of repeats containing approximately ~90-100 amino acids. Each BRCT repeat adopts a characterized fold with a central, parallel four-stranded β -sheet, along with a pair of α -helices packed against one face and a single α -helix packed against the opposite face of the sheet. The arrangement of the α 1, α 3 and the central β-sheet is conserved in all repeats as a number of key hydrophobic residues maintain the packing of the BRCT fold. The two BRCT repeats in BRCA1 interact in a head-to-tail manner. In this arrangement, the N-terminal half of the one BRCT domain forms a pocket for pSer as the C-terminal half of the same domain generates a hydrophobic pocket for Phe.
Structure Reference
- Shiozaki, E. N. et al. (2004) Mol. Cell. 14, 405–412.
- Clapperton, J.A. et al. (2004) Nature Struct. Biol. 11(6), 512–518.
- Williams, R. S. et al. (2004) Nature Struct. Biol. 11(6), 519–525.
Examples of Domain Proteins
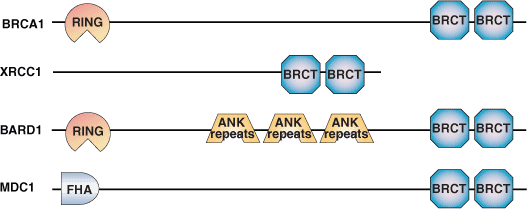
Binding Examples
BRCT Domain Proteins | Binding Partners | Phosphopeptide Ligands |
BRCA1 | BACH1, p53, CtIP, HDACs, CBP | pSer-X-X-Phe |
XRCC1 | LigIIIα |
|
XRCC4 | DNA Ligase IV |
|
TOPBP1 | E2F1 |
|
53BP1 | p53 |
|
RAD9 | RAD4 | pSer-[YILQP]-I-I |
BARD |
| pSer-[DE]-[DE]-E |
MDC1/NFBD1 | H2AX | pSer-Φ-[EVDI]-Ω |

