C1 Protein Domain
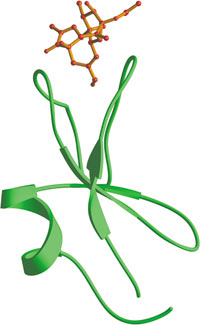
The C-terminal C1 domain of PKCδ bound to phorbol ester (red).
Domain Binding and Function
Cysteine-rich C1 domains are approximately 50 amino acids long and are involved in the recruitment of proteins to the membrane. Typically, C1 domains bind phorbol esters or diacylglycerol (DAG), which are necessary for membrane localization. When bound to phorbol ester, the upper surface of the C1 domain forms a contiguous hydrophobic surface in the domain. This enables the region to be buried into the lipid bilayer, which stabilizes membrane insertion. The middle portion of the domain contains a number of basic residues that can interact with membrane lipid head groups, while the lower half of the C1 domain contains two zinc-binding sites that are important in maintaining proper domain folding. The two major classes of C1 domain containing proteins are protein kinase C (PKC) isozymes and chimaerins. Chimaerins, or "non-kinase" phorbol ester receptors, are Rac-GAPs that are lipid-regulated via their C1 domain.
Structure Reference
- Zhang, G. et al. (1995) Cell 81(6), 917–924.
Examples of Domain Proteins
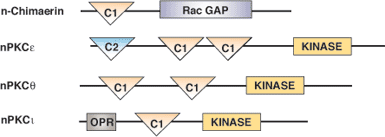
Binding Examples
C1 Domain Proteins | Binding Partners |
PKC Isoforms (classical and novel) | Diacylglycerol or phorbol esters |
Diacylglycerol Kinase | Diacylglycerol or phorbol esters |
c-Raf Ser/Thr Kinase | Diacylglycerol or phorbol esters |
n-Chimaerin Rac-GTPase Activating Protein | Diacylglycerol or phorbol esters |

