CC Protein Domain
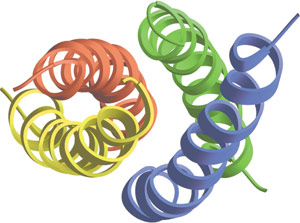
Coiled coils in the tetrameric Mnt repressor of Salmonella bacteriophage P22.
Domain Binding and Function
Coiled-coils (CC) function as oligomerization domains for a wide variety of proteins including structural proteins, motor proteins and transcription factors. The coiled-coil structure is conserved from viruses to plants and mammals and it has been predicted that approximately 5% of proteins encoded in sequenced genomes contain coiled-coils. Coiled-coils typically consist of two or more α-helices that wrap around each other with a superhelical twist. Sequences with a propensity to assume coiled-coil structures are characterized by the heptad repeat pattern (abcdefg)n, where a and d are hydrophobic amino acids and e and g are charged or polar. Coiled-coils may interact with each other to form homotypic oligomers or with other coiled-coil domains to form heterotypic oligomers.
Structure Reference
- Nooren, I.M. et al. (1999) Nat. Struct. Biol. 6(8), 755–759.
Examples of Domain Proteins
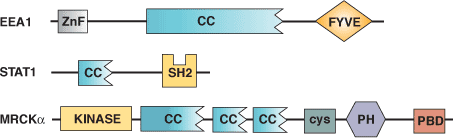
Binding Examples
CC Domain Proteins | Binding Partners |
EEA1 Early Endosome Protein | Homotypic and heterotypic interactions |
Stat1 Transcription Factor | Homotypic and heterotypic interactions |
Fos and Jun | Heterotypic interactions |

