CUE Protein Domain
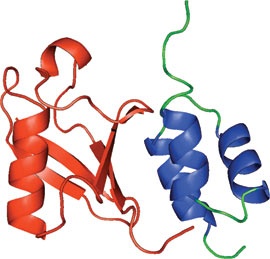
CUE domain of the yeast Cue2 protein bound to ubiquitin (red).
Domain Binding and Function
The CUE domain was first described as a region of homology between the yeast Cue1 (coupling of ubiquitin to ER degradation) and human Tollip proteins. The domain is approximately 40 amino acids in length and is found in proteins that have a variety of functions, including the degradation of misfolded proteins in the endoplasmic reticulum and protein sorting. CUE domains have been shown to both bind mono and polyubiquitin and some CUE domain-containing proteins are themselves ubiquitinylated in a CUE domain-dependent manner.
Structure
The CUE domain is structurally related to the ubiquitin-binding UBA domain and is comprised of a three-helix bundle. A conserved MFP motif in α−helix1 and an LL motif in α−helix 3 interact with the conserved hydrophobic patch of ubiquitin. The CUE domain has also been reported to exist as a domain-swapped dimer that makes additional contacts with ubiquitin, and consequently binds ubiquitin with a higher affinity.
Structure Reference
- Kang, R.S et al. (2003) Cell 113(5), 621–630
Examples of Domain Proteins
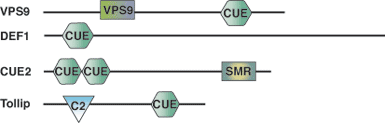
Binding Examples
CUE Domain Partners | Binding Partners |
Vps9 | mono and polyubiquitin |
Cue1 | mono and polyubiquitin |
Tollip | monoubiquitin (polyubiquitin not tested) |

