DED Protein Domain
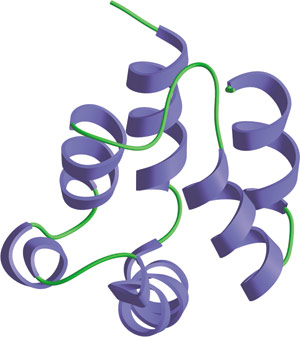
The DED domain in FADD.
Domain Binding and Function
The Death Effector Domain (DED) is a protein interaction domain found in inactive procaspases (cysteine proteases) and proteins that regulate caspase activation in the apoptosis cascade. Similar to CARDs, DEDs recruit procaspases into complexes with members of the TNF-receptor superfamily. This recruitment is mediated by a homotypic interaction between the procaspase DED and a second DED in an adaptor molecule that is directly associated with activated TNF receptors. Complex formation allows transprocessing of procaspase to the active form, which activates downstream caspases and initiates apoptosis.
Structure Reference
- Eberstadt, M. et al. (1998) Nature (6679)392, 941–945.
Examples of Domain Proteins
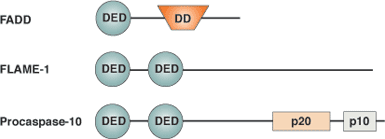
Binding Examples
DED Domain Proteins | Homotypic Binding Partners | Functions |
Procaspase-8 | FADD adaptor protein; Flame-1 | Activation of apoptosis by the Fas receptor |
Flame-1 (aka FLIP, I-FLICE, Usurpin, etc.) caspase activation inhibitor | FADD; Procaspase-8 | Inhibition of Procaspase-8 activation |

