EVH1 Protein Domain
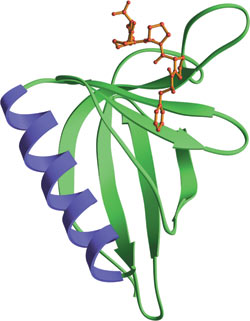
The EVH1 domain from EVL bound to ActA peptide (red).
Domain Binding and Function
The Ena/Vasp Homology domain 1 (EVH1) is a protein module of ~115 amino acids found in a number of scaffolding proteins that mediate the multiprotein complex assembly associated with control of the actin cytoskeleton and signal transduction in postsynaptic compartments. This domain was originally identified from the N-terminus of the Drosophila protein Enabled (Ena), its mammalian counterpart (Mena) and the closely related protein Vasp. EVH1 domains are also found in another Mena family protein (Evl), in the WASP docking protein altered in the immunodeficiency Wiskott-Aldrich syndrome, and in the Homer family of synaptic proteins that interact with group 1 metabotropic glutamate receptors. EVH1 domains are divided into two classes based on association with proline-rich ligands. Class I EVH1 domains include Ena/Vasp proteins that recognize an FPxΦP motif, while class II EVH1 proteins are postsynaptic, receptor-associated proteins that recognize PPxxF motifs. These motifs are commonly found in cytoskeletal proteins, such as Vinculin and Zyxin, and in the pathogenic bacterium Listeria monocytogenes ActA protein, which regulates bacterial motility by controlling host cell actin polymerization.
Structure Reference
- Federov, A.A. et al. (1999) Nat. Struct. Biol. 6(7), 661–665
Examples of Domain Proteins
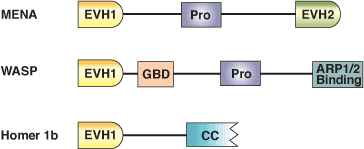
Binding Examples
EVH1 Containing Proteins | Binding Partners | Motif |
Class I Ena/VASP | Vinculin, Zyxin, ActA | FPxΦP |
Class II Homer-Ves1 | mGluR, IP3R, RyR | PPxxF |

