F-Box Protein Domain
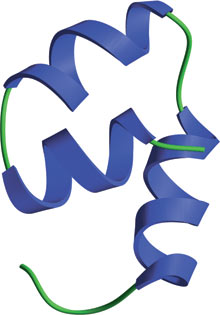
The F-Box domain from human Skp2.
Domain Binding and Function
The F-Box domain is a 42–48 conserved amino acid domain found at the N-terminus of F-Box proteins. F-Box proteins act as modular E3 ubiquitin ligase adaptor proteins within the SCF complex responsible for phosphorylation-mediated ubiquitination. The F-Box domain mediates interaction with SKP1, which links F-Box proteins to the core ubiquitin-ligase complex that is composed of Rbx1, cdc53/Cul1 and the E2 conjugating enzyme cdc34. The C-terminal region of F-Box proteins is also composed of various modular domains that interact with target substrates, often in a phosphorylation-dependent manner.
Structure Reference
- Schulman, B.A. et al. (2000) Nature 408(6810), 381–386.
Examples of Domain Proteins
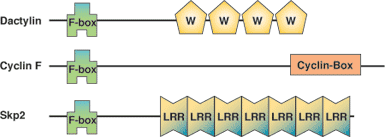
Binding Examples
F-Box Domain Proteins | Binding Partners | C-terminal Binding Partners |
Cdc4 (Yeast) | Skp1, Rbx1 | Sic1, CDK inhibitor |
Grr1 (Yeast) | Skp1, Rbx1 | Cyclin (CLN) 1,2 |
TrCp (Yeast) | Skp1, Rbx1 | IΚB, NF-ΚB regulator |

