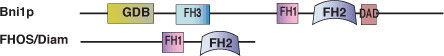
FH2 Protein Domain
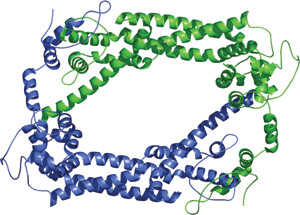
Tethered dimer of the FH2 domain from S. cerevisiae Bni1p.
Domain Binding and Function
The approximately 400 amino acid FH2 domain promotes filamentous actin growth by catalyzing the nucleation of actin chains. Proteins with FH2 domains induce unbranched actin filaments, in contrast to the Arp 2/3 complex that initiates branched actin filament formation. FH2 domains are found in many proteins that are required in a variety of cytoskeletal dependent processes such as cellular polarity, morphogenesis, and cytokinesis.
Structure
Formin Homology-2 (FH2) domains are approximately 400 residues in length with an almost entirely α helical structure that dimerize in a head to tail fashion. The resulting symmetric homodimer forms a parallelogram like structure when viewed from the top. The homodimers interact by a lasso at the N-terminus of one monomer wrapping around a protuberance from the post subdomain at the C-terminus of the other monomer. This lasso and post interface is thought to mediate actin binding. A linker extends from each lasso and tethers the monomers to each other.
Structure Reference
- Xu, Y. et al. (2004) Cell 116(5), 711–723.
Examples of Domain Proteins