FYVE Protein Domain
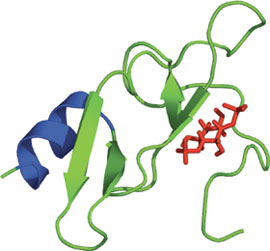
The FYVE Domain of EEA1 bound to PI(1,3)P2.
Domain Binding and Function
The FYVE (Fab-1, YGL023, Vps27, and EEA1) domain is a small, cysteine-rich Zn2+ binding domain of approximately 60-70 amino acids. To date, FYVE domains have been identified in over 300 different proteins from yeast to man. The FYVE domain structure consists of two β-hairpins plus a small C-terminal α-helix held together by two Zn2+ binding clusters. FYVE domains contain a basic motif in the first β-strand (R/K)(R/K)HHCR, creating a positively charged pocket that binds PI(3)P. Upon binding of the FYVE domain to PI(3)P, a membrane insertion loop (MIL) located within the domain penetrates into the phospholipid bilayer. The EEA1 protein has an increased affinity for PI(3)P upon dimerization as well as lowered cytosolic pH. FYVE domain binding of PI(3)P has implicated it in a signaling role downstream of PI(3)kinase. Furthermore, FYVE containing proteins have been implicated in the regulation of the vacuolar/lysosomal membrane trafficking pathway and in the regulation of signaling by TGFβ-receptors.
Structure Reference
- Kutateladze, T and Overduin, M. (2001) Science 291(5509), 1793–1796
Examples of Domain Proteins
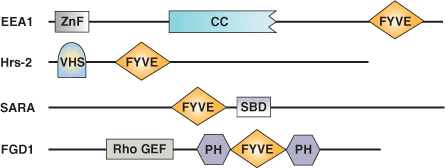
Binding Examples
FYVE Domain Proteins | Binding Partners |
EEA1 Early Endosome Antigen | PI(3)P |
Hrs Putative ATPase | PI(3)P |
SARA (Smad Anchor for Receptor Activation) | PI(3)P |
FENS1 | PI(3)P |

