GEL Protein Domain
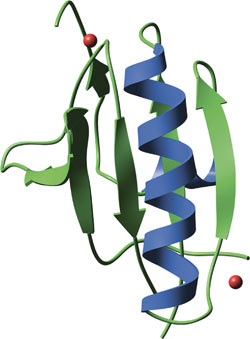
GEL domain from human plasma gelsolin, with bound calcium ions in red.
Domain Binding and Function
Also know as the gelsolin/severin/villin homology domain, the Gelsolin homology domain (GEL) is a 120–150 amino acid domain found in a number of proteins involved in cytoskeletal regulation and is particularly important in actin severing proteins. The GEL domain binds both calcium and actin, suggesting that the actin binding activity of GEL proteins is calcium regulated. GEL proteins typically bind to the barbed ends of actin filaments to prevent monomer exchange, acting as an end-blocking or capping protein for the actin filament. Proteins with gelsolin homology domains can promote actin nucleation to create new filaments and sever existing filaments.
Structure
Individual GEL domains consist of a central five- or six-stranded β-sheet sandwiched between two perpendicular α-helices. While individual GEL domains can bind actin, proteins such as gelsolin contain three GEL domains that act in concert for calcium-regulated actin binding. Calcium binding to GEL domains induces a conformational reorganization that cleaves the continuous β-sheet core of GEL4 and GEL5 to expose a GEL4 actin-binding site. Gelsolin contain two sets of triple-GEL domains, which binds two actin molecules in the severing and capping of actin filaments.
Structure Reference
- Robinson, R.C. et al. (1999) Science 286(5446), 1939–1942.
Examples of Domain Proteins
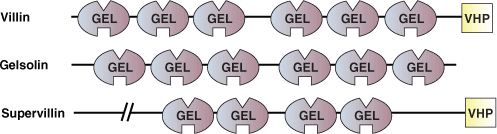
Binding Examples
GEL Domain Proteins | Binding Partners |
Gelsolin | Ca2+ and F-actin |

