GLUE Protein Domain
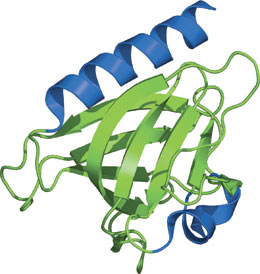
The GLUE domain of Vps36 (EAP45).
Domain Binding and Function
The GLUE (GRAM-Like Ubiquitin-binding in EAP45) domain structure has a typical PH domain architecture with two curved β sheets forming a barrel-like structure and one long α helix. Although the GLUE domain structurally resembles a split PH domain, the phosphoinositide pocket is clearly different than that of the PH domain. The major components of the endosomal sorting complex required for transport (ESCRT) complex are proteins recruited at different stages of the vesicular transport pathway. The GLUE domain plays a critical role in its ability to bind PtdIns3p and translocate Vps36 to endosomes. The affinity of the GLUE domain to bind PtdIns3p-containing vesicles is approximately 0.1μM. Mutations within the GLUE domain inhibit lipid binding and result in defects in the sorting of ubiquitinated cargo. Furthermore, the GLUE domain of Vps36 contains two integral NSF domains that recognize ubiquitin.
Structure Reference
- Teo, H. et al. (2006) Cell 125(1), 99–111.
Examples of Domain Proteins

Binding Examples
GLUE containing Proteins | Lipids Binding |
Vps36 | PtsIns3P, PtdIns4P, PtdIns(3,4)P2, PtdIns(3,5)P2 |

