PB1 Protein Domain
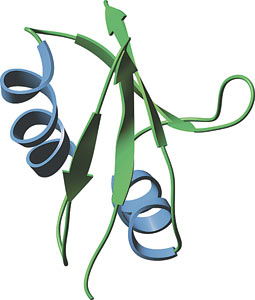
The PB1 domain from yeast Bem1.
Domain Binding and Function
Phox and Bem1 (PB1) domains contain approximately 80 amino acids and are found in a number of cytoplasmic signaling proteins. A PB1 domain may form heterodimers with a paired PB1 domain, although not all PB1 domains will associate with one another. A highly conserved internal sequence known as OPR, PC or AID motifs is necessary for PB1 domain function. Regions outside the OPR, PC and AID help confer specificity for binding.
Structure
One NMR structure of the PB1 domain of Bem1p has been solved. The PB1 domain consists of two α helices and a mixed, four-stranded β-sheet. The domain adopts a β-grasp fold similar to those found in ubiquitin and the Ras-binding domain of Raf. However, PB1 domains do not bind Ras related proteins, which suggests a functional distinction between PB1 and structurally similar Ras-binding domains.
Structure Reference
- Terasawa, H. et al., (2002) EMBO 20(15), 3947–3956.
Examples of Domain Proteins
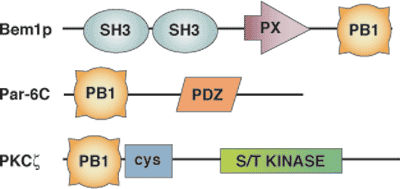
Binding Examples
PB1 Domain Proteins | Binding Partners |
Par-6 isoforms | PKCΖ, PKCΙ/Λ |
Bem1 | Cdc24 |
p67phox | p40phox |

