PDZ Protein Domain
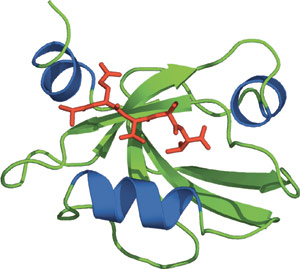
The third PDZ domain from PSD-95.
Domain Binding and Function
PDZ (Postsynaptic density 95, PSD-85; Discs large, Dlg; Zonula occludens-1, ZO-1) domains are one of the most frequently encountered domains, consisting of approximately 90 amino acid residues and identified as a region of sequence homology among a diverse list of signaling proteins. PDZ domains can occur in one or multiple copies and are nearly always found in cytoplasmic proteins. The PDZ domain consists of six β-strands and two α-helices, compactly arranged in a globular structure. Similar to PTB and PH domains, PDZ domains lack common peptide binding specificities and have the ability to interact with peptides and lipids. There are currently four different classes of PDZ interactions: recognition of carboxyl-terminal motifs in peptides, recognition of internal motifs in peptides, PDZ-PDZ dimerization, and recognition of lipids. Furthermore, PDZ domains that interact with C-terminal peptides can be divided into three separate classes according to their specificity.
Structure Reference
- Doyle, D.A. et al. (1996) Cell 85(7), 1067–1076.
Examples of Domain Proteins
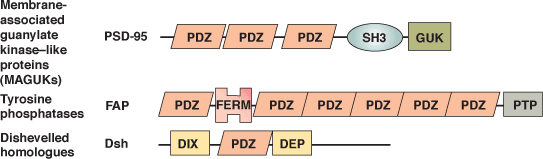
Binding Examples
PDZ Domain Proteins | Binding Partners | Class Type |
PSD-95 | NMDAR2A,B Shaker-type K+ channel | Class I: X-S or T-X-V |
NHERF or EBP50 | β2 –adrenergic receptor | Class I: X-S or T-X-L |
CASK | Neurexin, Syndecan | Class II: X-Ψ-X-Ψ |
nNOS | Melatonin Receptor | Class III: X-D or E-X-Ψ |

