POLO-Box Protein Domain
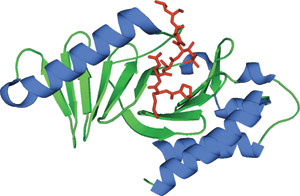
The PLK1 PBD bound to phospho-threonine peptide (red).
Domain Binding and Function
The POLO-Box domain is exclusively found in the family of Polo-like kinases that regulate mitotic entry, spindle assembly, centrosome maturation, chromosome segregation, cytokinesis and cell cycle arrest. The approximately 200 amino acid POLO-Box domain consists of two highly conserved Polo Box motifs of ~70-80 amino acids each, and is positioned C-terminal to an N-terminal Ser/Thr kinase domain. The POLO-Box domain performs dual roles in determining subcellular localization and autoinhibitory regulation of the kinase domain. The POLO-Box module consists of two POLO-Box motifs, the region between them, and a portion of the linker between the end of the kinase domain and the first POLO-Box. This module recognizes phosphopeptides with the core consensus motif Ser-pThr/pSer-Pro/X. Furthermore, PLK1 is overexpressed in a broad range of human tumors suggesting a role in carcinogenesis.
Structure
The POLO-Box domain consists of two β6α motifs that comprise of two POLO-Box regions (PB1 and 2). The two POLO-Box motifs form a 12-stranded β sandwich flanked by three α-helical segments. Though both POLO-Boxes comprise of a six-stranded antiparallel β-sheet shielded by one α-helix, the two POLO-Boxes exhibit only 12% sequence identity. The phosphopeptide interacts with β1 from PB1, the N-terminal end of L2 and β8/9 from PB2. The only residues that interact with the phosphate group are His-538 and Lys-540 from PB2. These phosphopeptide interacting residues are highly conserved in the POLO like kinases, suggesting a conserved ability to bind phosphopeptide motifs across all family members.
Structure Reference
- Cheng, K.Y. et al. (2003) EMBO 22(21), 5757–5768.
- Elia, A. et al. (2003) Cell 115(1), 83–95.
Examples of Domain Proteins
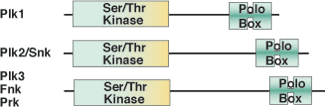
Binding Examples
POLO like Kinase | Binding partners |
Plk1 | Cdc25C, Chk2, Mcm7, GRASP65 |
Consensus Binding Sites
[Pro/Phe]-[φ/Pro]-[φ/Ala/Gln]-[Thr/Gln/His/Met]-Ser-[pThr/pSer]-[Pro/X]

