RING Protein Domain
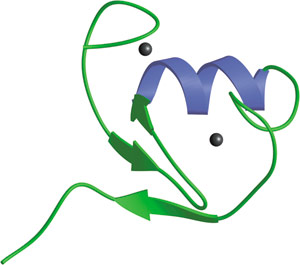
The RING domain in c-Cbl.
Domain Binding and Function
The 40-60 residue RING finger domain is a specialized type of zinc finger that binds a pair of zinc atoms and is involved in mediating protein—protein interactions. Presence of a RING finger domain is characteristic of RING-class E3 ubiquitin protein ligases that transfer ubiquitin from an E2 enzyme to a substrate protein. The RING domain mediates the interaction with the appropriate E2 enzyme. Unlike HECT E3 ligases that form a thioester intermediate with ubiquitin, RING fingers likely mediate ubiquitination by facilitating the direct transfer of ubiquitin from E2 enzymes to lysine residues on the target substrate. RING finger proteins include the Hrt1/Roc1/Rbx1 proteins found in both the SCF and VCB-like E3 complexes, the APC1 component of the Anaphase Promoting Complex, Cbl family proteins, MDM2 and many other proteins with demonstrated E3 ligase activity, E2 binding or involvement in ubiquitination. RING finger domains are also associated with certain transcription factors, including TIF1β, the PML-family, NFX1 and XPRF.
Structure Reference
- Zheng, N. et al. (2000) Cell 102(4), 533–539.E
Examples of Domain Proteins
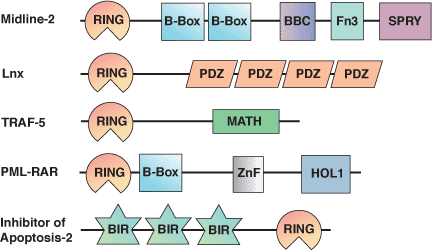
Binding Examples
RING Domain Proteins | Binding Partners |
Cbl | UbcH7 |
RAD5 | UBC13-MMS2 complex |
RAD6 | RAD18 |
HHARI | UbcH7 |

