SPRY Protein Domain
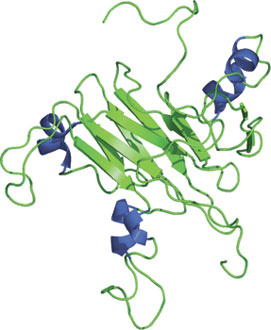
Structure of the SPRY domain of SSB-2.
Domain Binding and Function
The SPRY domain was originally discovered as a sequence-repeat in the dual-specificity kinase splA of Dictyostelium and rabbit ryanodine receptor. The ~140 amino acid residue domain adopts a novel fold consisting of a β-sandwich structure formed by two four-stranded antiparallel β-sheets with a unique topology. In combination with the B30.2 domain, these two domains adopt an immunoglobulin-like fold. The SPRY of SSB-2 binds prostate apoptosis response protein-4 (Par-4), while the SPRY domains of RanBPM, RanBP10 and SSB-1 mediate interactions with MET. Mutations found in the SPRY-containing proteins have shown to cause Mediterranean fever and Opitz syndrome.
Structure Reference
- Masters, S. L. (2006) Nature Struc. & Mol. Biology 13(1) 77–84.
Examples of Domain Proteins
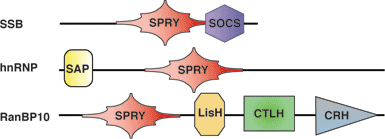
Binding Examples
SPRY Proteins | Binding Partner |
SSB1, SSB2, SSB-4 | Par-4 |
RanBPM, RanBP10 | c-Met |
Pyrin (Marenostrin) |

