UIM Protein Domain
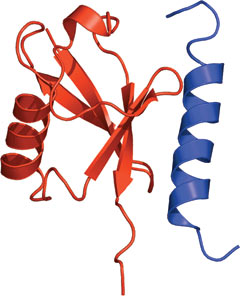
The UIM domain of Vps27 bound to ubiquitin (red).
Domain Binding and Function
The Ubiquitin-Interacting Motif (UIM) was first described as a region homologous to the ubiquitin binding S5a subunit of the 26S proteasome. The motif can bind monoubiquitin as well as polyubiquitin chains and is found in a number of proteins involved in endocytosis and protein trafficking. The binding of UIM to monoubiquitylated substrates may limit polyubiquitin chain assembly by blocking subsequent ubiquitin attachment to Lys48 of ubiquitin. Interestingly, several UIM domain-containing proteins are themselves ubiquitylated in a UIM domain-dependent manner.
Structure
The UIM domain consists of a single α helix with hydrophobic residues within the helix interacting with the conserved Leu8-Ile44-Val70 hydrophobic patch of ubiquitin. Acidic residues within the UIM also contribute to binding through interaction with basic residues in ubiquitin.
Structure Reference
- Swanson, K.A., et al. (2003) EMBO J. 22(18) 4597–4606.
Examples of Domain Proteins
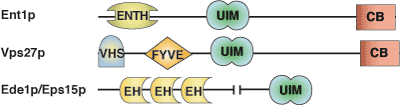
Binding Examples
UIM Domain Proteins | Binding Partners |
HRS | mono- and polyubiquitin |
Epsin | mono- and polyubiquitin |
Eps15 | mono- and polyubiquitin |

