NOTE: For the best possible results, Cell Signaling Technology (CST) strongly recommends using our optimized application-specific protocols for each product. These protocols are the result of extensive in-house validation performed at CST and ensure accurate and reproducible results.
Detection of western blots using fluorescent-conjugated secondary antibodies can allow for more accurate quantification and two-color multiplexing using primary antibodies from different species. Phosphorylation and total protein levels may be determined on the same membrane, and regulation of different targets at different molecular weights can be examined simultaneously. It is important to remember, however, that primary antibody affinities vary, and no two individual antibodies can be directly compared to one another, but must be standardized. In some instances, fluorescent detection may not be as sensitive as chemiluminescent detection and therefore may not be suitable for proteins with low expression levels. Fluorescent western detection requires specialized instrumentation, such as a fluorescent imager.
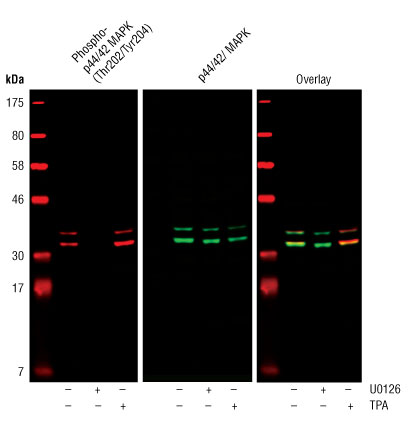
Western blot analysis of extracts from COS cells, untreated or treated with either U0126 (#9903) (10 µM for 1h) or TPA (#4174) (200 nM for 10m), using Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP® Rabbit mAb (#4370) and p44/42 MAPK (Erk1/2) (3A7) Mouse mAb (#9107).
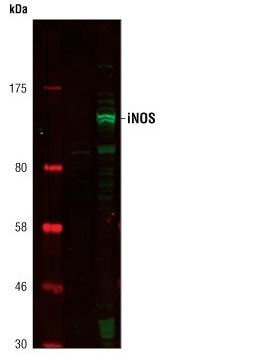
Western blot analysis of extracts from Raw264.7 cells, untreated or LPS-treated (1 µg/ml for 6 h), using iNOS Antibody (#2977).
CST offers a modified immunoblotting protocol for use in fluorescent western blotting. We find that omitting Tween-20 during the blocking step is the only significant change necessary. Full details can be found in our Fluorescent Western Immunoblotting Protocol (Primary Ab Incubation In BSA) and our Fluorescent Western Immunoblotting Protocol (Primary Ab Incubation In Milk). Additionally, our Prestained Protein Marker, Broad Range (Premixed Format) #7720 is autofluorescent at near-infrared wavelengths.
Two-color Western blots require primary antibodies from different species and appropriate secondary antibodies labeled with different dyes. If the primary antibodies require different primary antibody incubation buffers, each primary antibody should be tested individually in both buffers to determine which will be optimal for the dual-labeling experiment. Some antibody pairs may not work together due to overlapping epitopes, interference caused by primary and/or secondary antibodies, or incompatibilities in primary antibody incubation buffers.