Adhesion/ECM/Cytoskeleton
CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
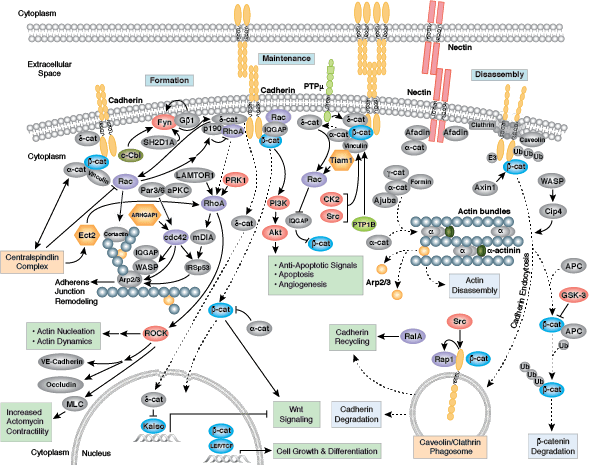
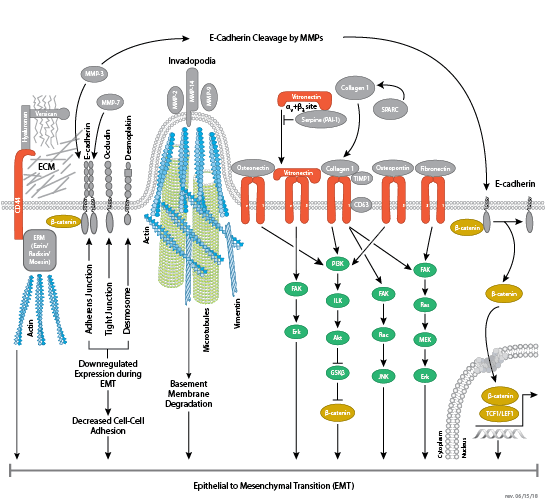
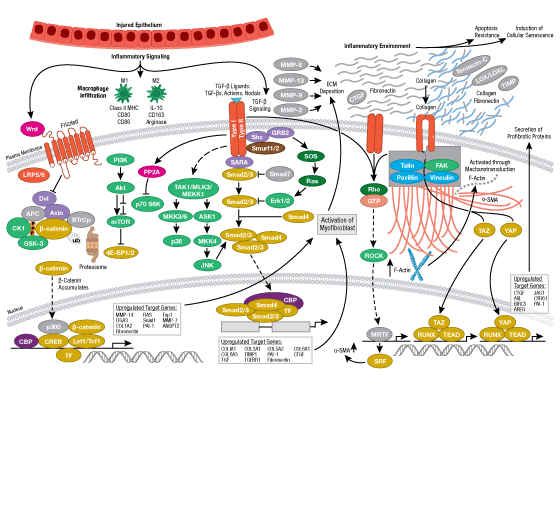
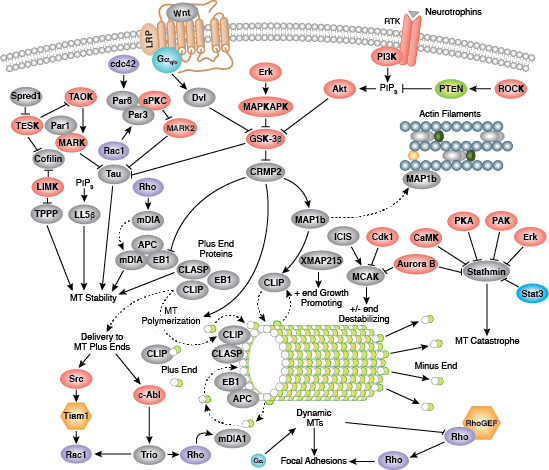
Cells can form a number of connections with the cells and matrix in their surrounding environment: adherens junctions (cell-cell), tight junctions (impermeable cell-cell), and focal adhesions (cell-matrix).
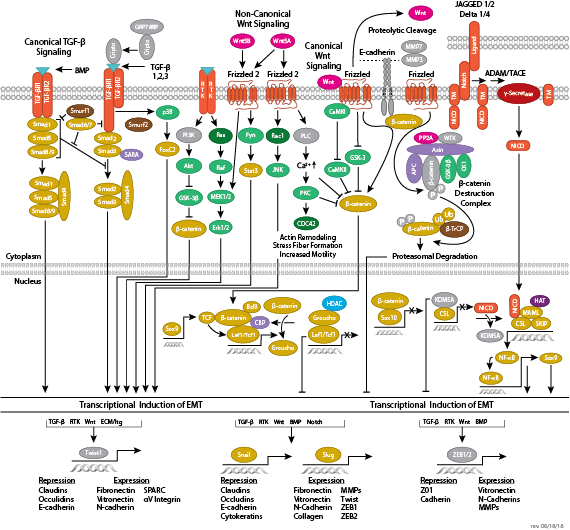
Adherens junctions are dynamic structures that form, strengthen and spread, degrade, and then re-form as their associated proteins create ephemeral connections with counterparts from adjacent cells. Formation of adherens junctions are mediated through a cadherin-catenin complex composed of cadherin, β-catenin, and α-catenin. The connection between cell junctions and the cytoskeleton may be more dynamic than originally considered and may rely on multiple, weak associations between the cadherin-catenin complex and the actin cytoskeleton or rely on other membrane-associated proteins (i.e. nectin and afadin). Monomeric α-catenin binds β-catenin at adherens junctions and upon release, forms α-catenin dimers that promote actin bundle formation. The transition from branched actin networks to bundled actin filaments correlates with the creation of mature, strong adherens junctions and a decrease in membrane lamellipodia. As with most dynamic cellular systems, a collection of kinases, phosphatases, and adaptor proteins regulate the activity and localization of a few key effector proteins. p120 catenin (δ-catenin) binds and stabilizes cadherin at the plasma membrane. Membrane-bound and cytosolic tyrosine kinases phosphorylate β-catenin at weak or nascent junctions, while phosphatases remove added phosphates from β-catenin and δ-catenin at established junctions. Rho family GTPases modulate the availability and activation state of catenins and other essential adherens proteins. Together, this collection of structural proteins, enzymes, and adaptor proteins create dynamic cell-cell junctions necessary for temporary associations during morphogenesis and maintains the integrity of complex tissues and structures following development.
Tight junctions are impermeable cell-cell junctions that form a continuous barrier to fluids across the epithelium and endothelium. They function in regulation of paracellular permeability and in the maintenance of cell polarity, blocking the movement of transmembrane proteins between the apical and the basolateral cell surfaces. Tight junctions are composed of claudin and occludin transmembrane proteins, which join the junctions to the cytoskeleton. Occludin is thought to be important in the assembly and maintenance of tight junctions. Differential phosphorylation of occludin at various residues may regulate its interaction with other tight junction proteins such as ZO-1.
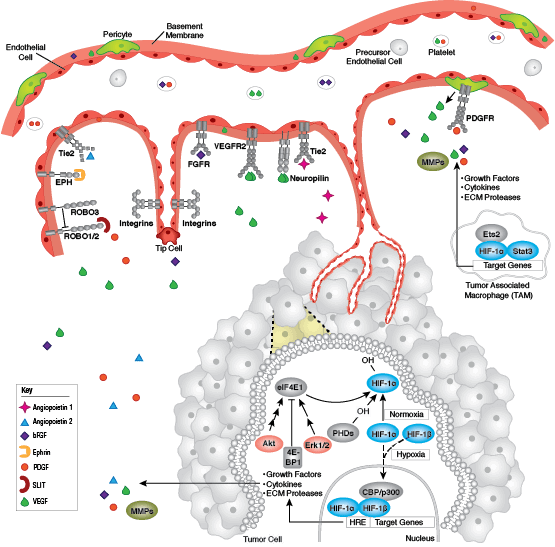
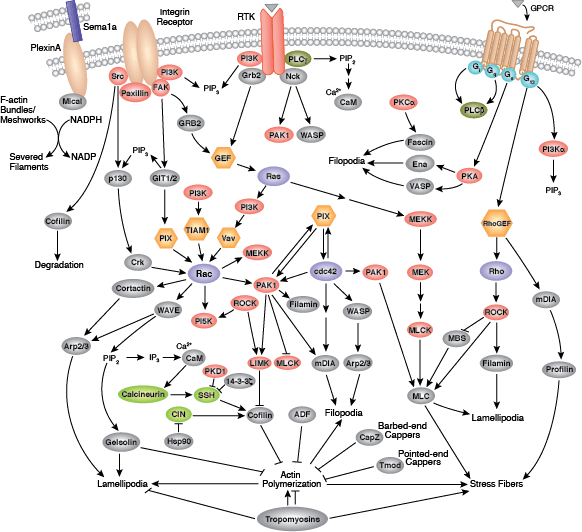
Focal adhesions are the connections that form between a cell and its extracellular matrix and are mediated primarily through integrins. Integrins are α/β heterodimeric cell surface receptors that play a pivotal role in cell adhesion and migration. The integrin family contains at least 18 α and 8 β subunits that form 24 known integrins with distinct tissue distribution and overlapping ligand specificities. The intracellular tail of integrins interact with numerous signaling molecules including focal adhesion kinase (FAK). Activation of FAK by integrin clustering leads to autophosphorylation at Tyr397, which is a binding site for the Src family kinases PI3K and PLCγ.
References:
- Gates J, Peifer M (2005) Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell 123(5), 769–72.
- Perez-Moreno M, Fuchs E (2006) Catenins: keeping cells from getting their signals crossed. Dev. Cell 11(5), 601–12.
- Fox DT, Peifer M (2007) Cell adhesion: separation of p120's powers? Curr. Biol. 17(1), R24–7.
- Yap AS, Crampton MS, Hardin J (2007) Making and breaking contacts: the cellular biology of cadherin regulation. Curr. Opin. Cell Biol. 19(5), 508–14.
- Niessen CM, Gottardi CJ (2008) Molecular components of the adherens junction. Biochim. Biophys. Acta 1778(3), 562–71.
- Nelson WJ (2008) Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem. Soc. Trans. 36(Pt 2), 149–55.
- Schmalhofer O, Brabletz S, Brabletz T (2009) E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 28(1-2), 151–66.
- Meng W, Takeichi M (2009) Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol 1(6), a002899.