Immunology/Inflammation
CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
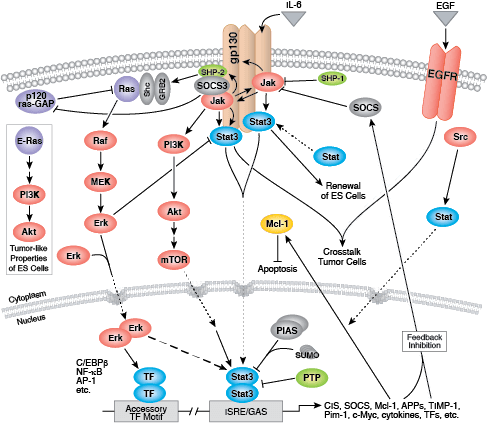
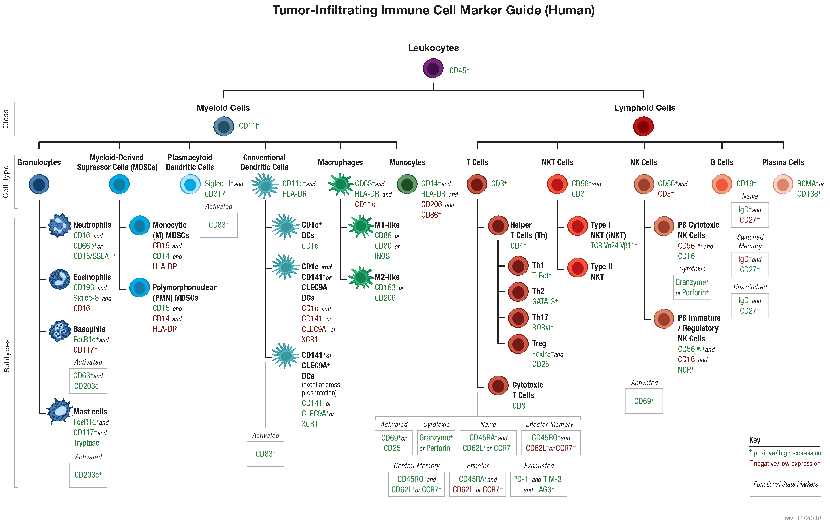
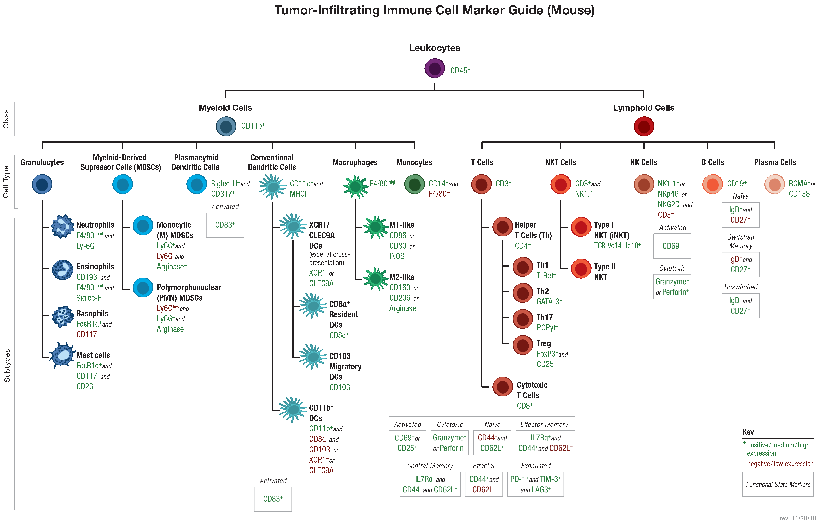
B and T lymphocytes mediate the humoral and cell-mediated immune responses, respectively, which make up the adaptive arm of the immune system. B cells mature in the bone marrow and differentiate into antibody-secreting plasma cells. In contrast, T cells are thymus-derived and as effector cells, orchestrate cell-mediated immunity.
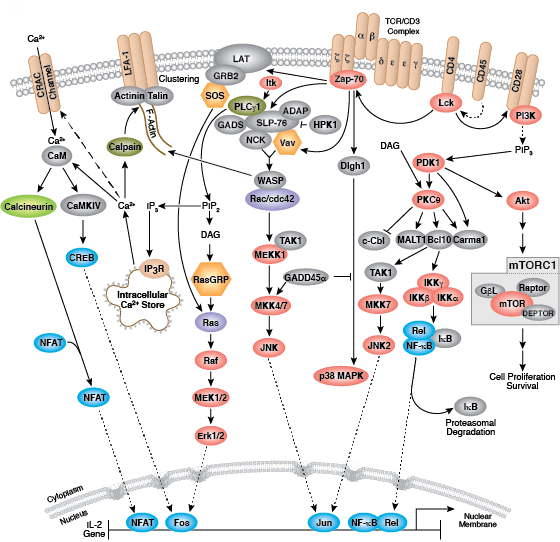
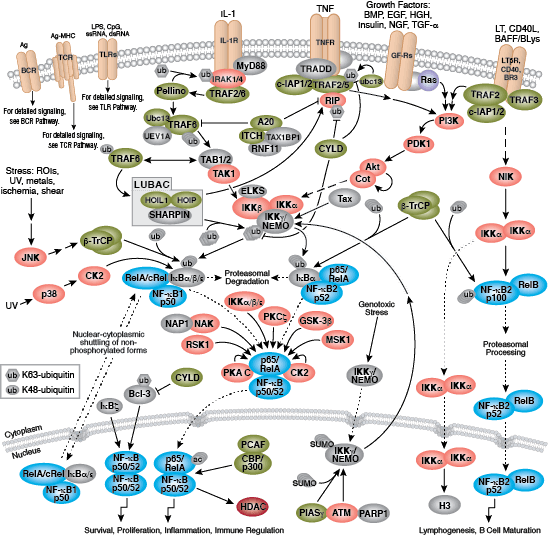
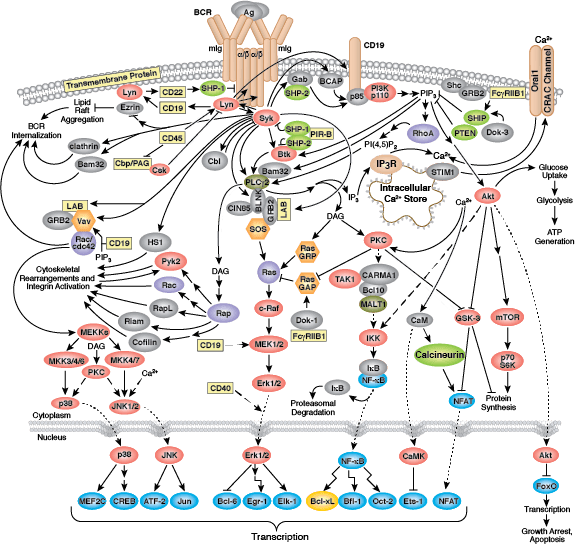
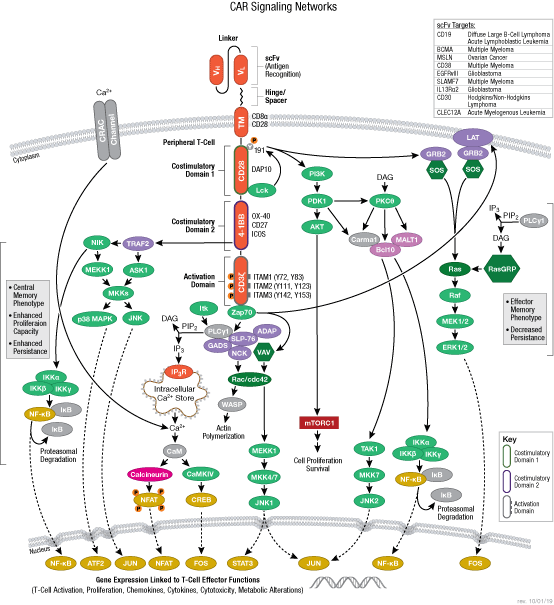
The B cell receptor (BCR) is composed of a membrane-bound antibody (immunoglobulin or Ig) flanked by Igα/Igβ (CD79A/CD79B) heterodimers. While membrane Ig binds antigen, the CD79 heterodimer transduces signals through its cytoplasmic immunoreceptor tyrosine- based activation motif (ITAM) domains. The T cell receptor (TCR) consists of a membrane-bound αβ heterodimer (TCRαβ), four CD3 chains (two CD3ε, one CD3γ, one CD3δ), and a ζ−chain homodimer. The TCRαβ dimer recognizes antigenic peptides, while the associated signaling chains transduce signals with their cytoplasmic ITAM domains. Thus, the lymphocyte antigen receptors use similar models of membrane-bound antigen receptors linked to signal-transducing accessory chains.
Signaling through the BCR and TCR involves activation of a number of Src family tyrosine kinases (Blk, Fyn, and Lyn in B cells and Fyn and Lck in T cells), which are responsible for phosphorylation of the receptor-associated ITAM motifs. Phosphorylated ITAMs act as docking sites for Syk family tyrosine kinases (Syk in B cells and Zap-70 in T cells). Activated Syk kinases amplify signals through phosphorylation of downstream adaptor proteins, thereby initiating a cascade of intracellular signaling molecules. In addition to mediating cell activation, lymphocyte receptor signaling drives B and T cell development, differentiation, proliferation, and survival.
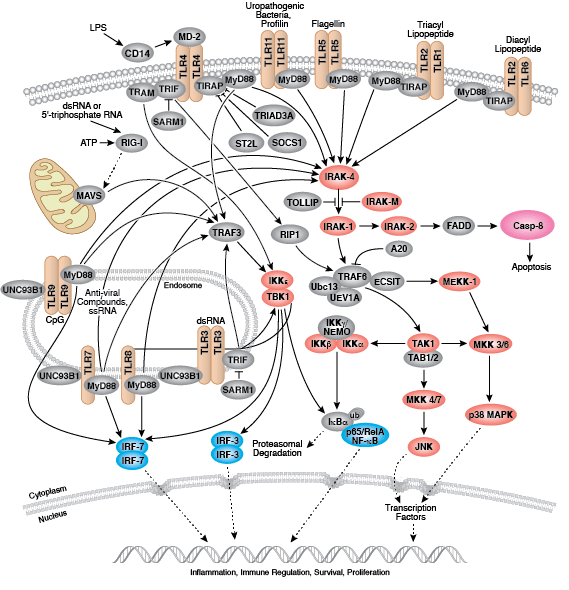
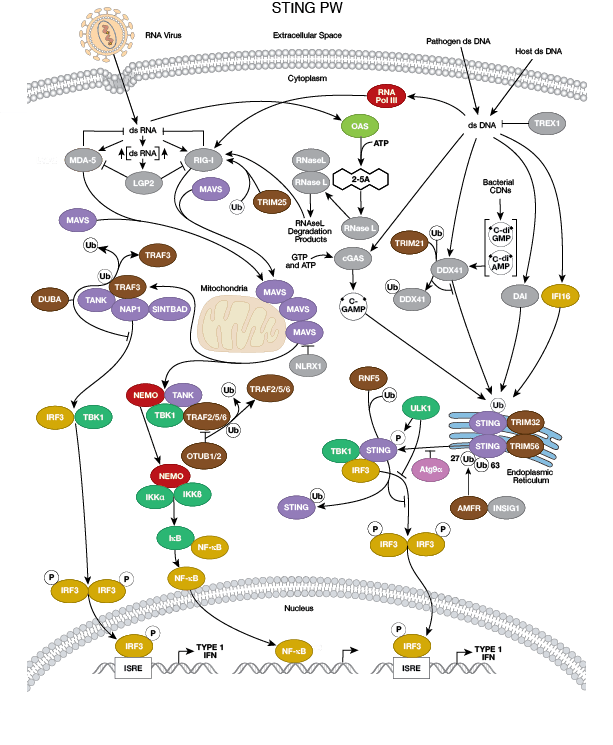
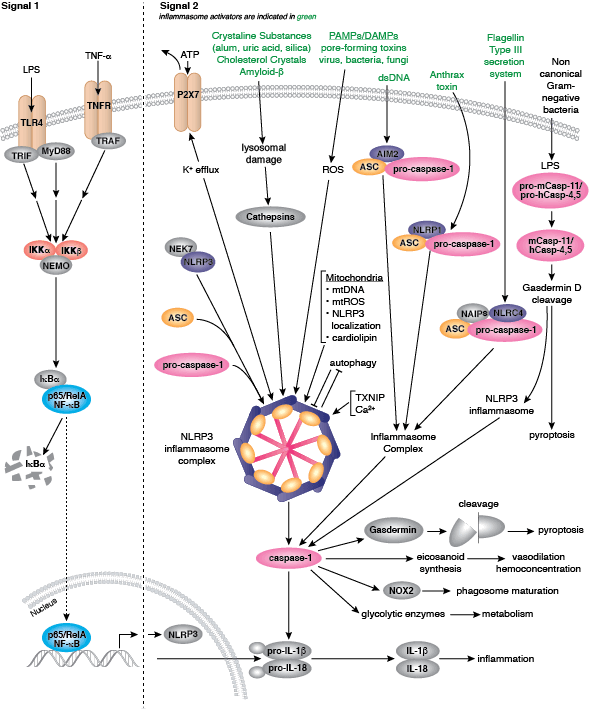
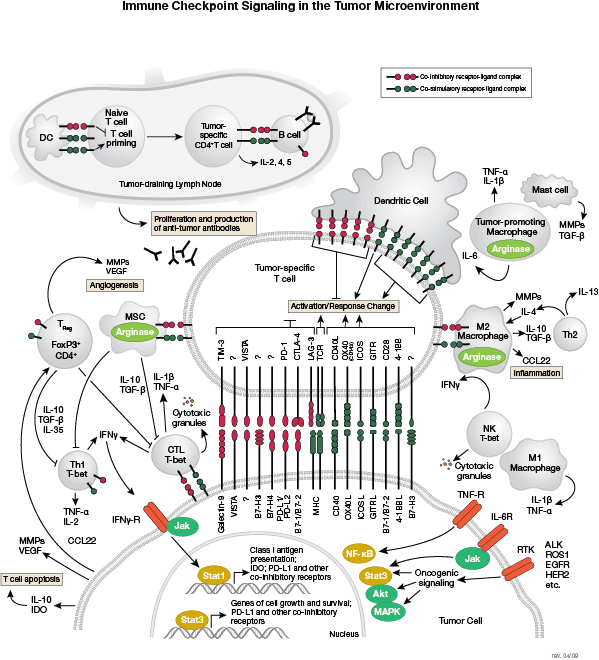
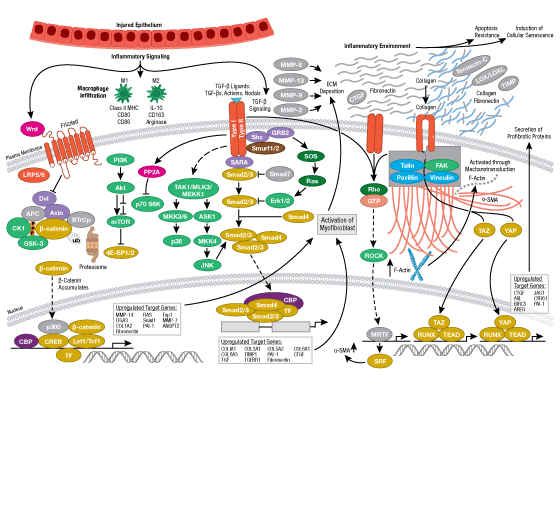
The innate arm of the immune system consists of a host of immune cells and resistance mechanisms that act as the first line of defense against invading pathogens. The Toll-like receptors (TLRs) are a family of evolutionarily conserved pattern recognition receptors (PRRs) that recognize the pathogen-associated molecular patterns (PAMPs) found in microbial pathogens. TLR1, 2, 4, 5, and 6 are expressed at the cell surface, while TLR3, 7, 8, and 9 have been shown to localize to intracellular vesicles. Activation of TLRs through ligand binding triggers a signaling cascade involving a variety of intracellular signaling adaptors including MyD88, IRAKs, and TRAF6. TLR signaling leads to the activation of the MAP kinase, NF-κB, and IRF signaling pathways, which mediate inflammation through the production of inflammatory cytokines, type I IFN, chemokines, and antimicrobial peptides. TLR signaling in innate immune cells, particularly dendritic cells leads to their activation and subsequent induction of adaptive immune responses.
References:
- Harwood NE, Batista FD (2010) Early events in B cell activation. Annu. Rev. Immunol. 28, 185–210.
- Billadeau DD (2010) T cell activation at the immunological synapse: vesicles emerge for LATer signaling. Sci Signal 3(121), pe16.
- Maddaly R, Pai G, Balaji S, Sivaramakrishnan P, Srinivasan L, Sunder SS, Paul SF (2010) Receptors and signaling mechanisms for B-lymphocyte activation, proliferation and differentiation--insights from both in vivo and in vitro approaches. FEBS Lett. 584(24), 4883–94.
- Fuller DM, Zhu M, Ou-Yang CW, Sullivan SA, Zhang W (2011) A tale of two TRAPs: LAT and LAB in the regulation of lymphocyte development, activation, and autoimmunity. Immunol. Res. 49(1-3), 97–108.
- Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34(5), 637–50.
- Moresco EM, LaVine D, Beutler B (2011) Toll-like receptors. Curr. Biol. 21(13), R488–93.