CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
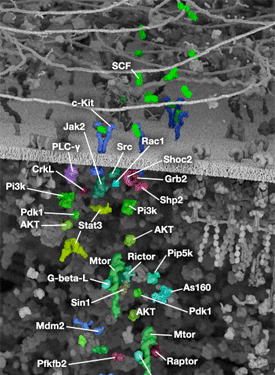
Two key events of translational control are the association between 5' capped mRNA and the pre-initiation complex, and the binding of initiator tRNA to the start codon. Both events are mediated by multiple eukaryotic initiator factors (eIF) that are regulated by effector kinases and inhibitors.
Formation of the eIF4F cap-binding complex precedes the association between mRNA and the pre-initiation complex. Subunit proteins eIF4A, eIF4G, and eIF4E comprise the eIF4F complex that binds the 5' mRNA cap structure, resolves mRNA secondary structures, and promotes pre-initiation complex formation. Assembly of eIF4F is controlled by growth and survival factors that regulate activity of upstream kinase effectors, including Akt, PI3K, p70 S6 kinase, p90RSK, and mTOR. mTOR kinase complexes mTORC1 and mTORC2 promote eIF4F cap-binding complex formation by activating upstream elements that favor complex assembly and inhibiting proteins that block eIF4F formation. The mTORC1 complex includes mTOR kinase bound by the adaptor Raptor and several regulatory proteins (Gβl, PRAS40, and DEPTOR), while the mTORC2 complex contains Rictor, Gβl, DEPTOR, Sin1, and mTOR kinase. mTORC1 activates p70 S6 kinase to relieve PDCD4 inhibition of eIF4A RNA helicase and activate eIF4B. Initiation factor eIF4B interacts with both the eIF3 scaffold protein complex and eIF4A, stimulating eIF4A RNA helicase activity. Upstream kinase pathways mediate the phosphorylation of eIF4B by p70 S6 kinase and p90RSK to increase the association between eIF4B, eIF3, and eIF4A. Inactivation of the eIF4E inhibitor 4E-BP1 by mTORC1 causes release of eIF4E and incorporation of cap-binding protein eIF4E into eIF4F. Stimulation of Akt by mTORC2 inhibits TSC2/TSC1, a heterodimer that indirectly inhibits mTOR activity through the small GTPase Rheb.
Binding of initiator tRNA to the ribosome at the start codon to form the 43S pre-initiation complex is mediated by eIF2. Trimeric eIF2 is made up of regulatory (α), tRNA/mRNA interacting (β), and GTP/GDP binding (γ) proteins. Phosphorylation of eIF2α by multiple upstream kinases (including PKR, PERK, and GCN2) follows environmental stress and the presence of dsDNA and leads to inactive eIF2 and translation inhibition. Additional control of eIF2 activity occurs through regulation of guanine nucleotide exchange, which is catalyzed by eIF2B. Exchange of GDP for GTP promotes the essential association between the eIF2 complex and tRNA. eIF2B activity is inhibited by GSK-3β phosphorylation and through interaction with eIF5, which also acts as a GDP dissociation inhibitor by stabilizing eIF2 bound by GDP.