SH2 Protein Domain
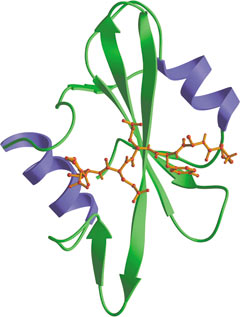
Src SH2 domain bound to phosphotyrosine peptide (red).
Domain Binding and Function
Src-homology 2 (SH2) domains are modules of approximately 100 amino acids that bind to specific phospho (pY)-containing peptide motifs. Conventional SH2 domains have a conserved pocket that recognizes pY, and a more variable pocket that binds 3-6 residues C-terminal to the pY to confer specificity. The SAP SH2 domain recognizes Y as well as pY in the context of residues N and C terminal, suggesting that an alternate 3-pronged model may sometimes apply. Phosphopeptides of optimal sequence bind to SH2 domains with dissociation constants of ~50-500 nM.
Structure Reference
- Waksman, G. et al. (1993) Cell 72(5): 779–90.
Examples of Domain Proteins
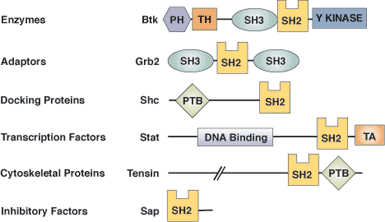
Binding Examples
SH2 Domain Proteins | Binding Partners | Phosphopeptide Ligand | SH2 Specificity Residues | |
|
| Regulation | Specificity |
|
Src Tyrosine Kinase | Focal Adhesion Kinase | pTyr | -Ala-Glu-Ile | Tyr ΒD5 |
Phospho-lipase C-γ C-terminal SH2 | PDGF β receptor | pTyr | -Ile-Ile-Pro-Leu-Pro-Asp | Cys ΒD5 |
Grb2 adaptor | Shc docking protein | pTyr | -Val-Asn-Val | Trp EF1 |

