VHL Protein Domain
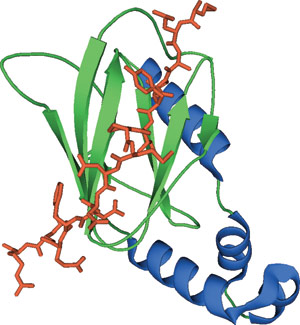
The VHL domain bound to a hydroxylproline peptide of HIFα.
Domain Binding and Function
VHL complexes with ElonginC, ElonginB, Cul2, and Rbx1 to form an E3 ubiquitin-protein ligase in which VHL acts as the substrate recognition subunit. The β domain of VHL is approximately 100 amino acids and has been shown to interact with hydroxylated prolines within target proteins. Contact residues in the β domain of VHL are well conserved in human, fly, frog and worm VHL orthologs. Substrates are subsequently polyubiquitinated and targeted for proteasomal degradation. The VHL β domain has been shown to interact with specific hydroxylated prolines of HIF-1α and the large subunit of RNA polymerase II. The VHL β domain has also been shown to interact with several atypical PKC isotypes.
Structure
The β-domain of the von Hippel-Lindau tumor suppressor protein (VHL) is composed of an amino-terminal, seven-stranded β sandwich and a carboxy-terminal α helix held against one of the β strands through hydrophobic interactions. The β sandwich forms a partially exposed hydrophobic core that, along with other polar residues, provides the binding site for the hydroxyproline peptide-containing ligand. This interaction is mediated by multiple van der Waals contacts between Trp88, Tyr98, and Trp117 of VHL to the pyrrolidine ring and hydrogen bonds between His115 to the 4-hydroxyl group of the hydroxyproline.
Structure Reference
- Min, J. et al., (2002) Science 296(5574), 1886–1889.
Examples of Domain Proteins

Binding Examples
VHL Domain Proteins | Binding Partners |
Hrs | Hrs FYVE domain |
