WD40 Protein Domain
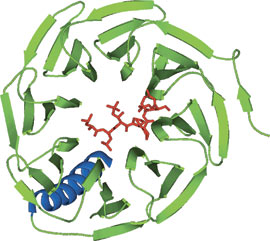
WD40 domain of Cdc4 bound to a CPD phosphopeptide from cyclin E.
Domain Binding and Function
WD40 repeats are among the most abundant of identifiable protein domains, found in a wide variety of eukaryotic proteins that perform functions such as adaptor/regulatory modules in signal transduction, pre-mRNA processing, cytoskeleton assembly, transcriptional activation and cell cycle control. A common theme among WD40 domain containing proteins is the ability of repeats to form β-propeller structures that act as a platform for the stable or reversible association of binding partners. The defining characteristics of WD repeats include conserved tryptophan (W) and aspartic acid (D) residues and a repeat length of approximately 40 amino acids. The WD40-repeat domain propeller structure is a versatile module involved in recognition of post-translational modifications. The WD40-repeat domains of β-TRCP and Cdc4 recognize phosphorylated serine and threonine containing peptides. In contrast, the WD40-repeat domain of WDR5 recognizes a dimethylated lysine peptide in the N-terminal tail of histone H3.
Structure
The WD40 repeat region from Cdc4 exhibits eight WD40 repeat regions that form an eight-bladed β-propeller structure. Most WD40 proteins with solved structures exhibit a seven-bladed β-propeller structure. The WD40 domain has been described as a conical frustum with a 40 Å diameter top surface, a 50 Å bottom surface, an overall thickness of 30 Å, and a central pore of 6 Å diameter. The WD40 domain recognizes the Cdc4 phosphodegron (CPD) peptide using a surface on top surface of the frustum.
Structure Reference
- Orlicky S., et al. (2003) Cell 112(2) 243–256.
Examples of Domain Proteins

Binding Examples
WD40 Containing Proteins | Target Proteins | Concensus |
βTRCP/Fbw1 | β-catenin, IκB | D-pS-G-φ-X-pS |
Cdc4/Fbw7 | Cyclin E, Sic1p, Gcn4p | L-I/L/P-pT-P |
WDR5 | Histone H3 | R-X-Kdimethyl |
