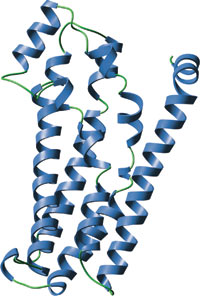
The DH domain of mouse TIAM-1.
The Dbl homology (DH) or RhoGEF domain consists of an ~ 150 amino acid region that induces Rho family GTPases to displace GDP. This effectively activates the Rho GTPase by allowing GTP binding, which is in excess over GDP in the cell. The DH domain is invariably preceded by a pleckstrin homology (PH) domain. While not absolutely required for catalysis of nucleotide exchange, the PH domain appears to greatly increase catalytic efficiency in many cases. Rho proteins control actin dynamics, gene expression, membrane trafficking, growth factor signaling, and cellular transformation. Proteins encoding DH domains (RhoGEFs) also play a role in these events by acting as primary Rho GTPase activators. Many RhoGEFs were identified based on their transforming activity, which was abrogated following disruption of their DH domain.
The DH domain is composed of a unique, extended bundle of α-helices. This bundle is composed mainly of the CR1, CR2, and CR3 regions that each form separate α−helices which pack together. The CR1 and CR3 regions are solvent exposed until complexed with a Rho GTPase where they then form the primary points of contact. Upon binding of the DH domain of Tiam-1 to Rac-1 the switch I region within the GTP binding pocket of Rac-1 is shifted and binds within a groove formed by the CR1 and CR3 regions within Tiam-1. The switch II region within Rac-1 binds to an even larger area of the DH domain causing residues 59 and 64 in Rac-1 to be displaced. The net effect of these changes is that the Mg2+ ion coordinated to GDP and the P loop which binds the terminal phosphate on GDP is displaced, which subsequently destabilizes the binding of GDP to Rac-1.
| DH Domain Proteins | Binding Partners |
| Dbl | RhoA/Rac1/Cdc42 |
| p190 RhoGEF | RhoA |
| Tiam-1 | Rac1/Cdc42 |
| p115 RhoGEF | RhoA |