PI3K/AKT/MAPK Signaling Resources
CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
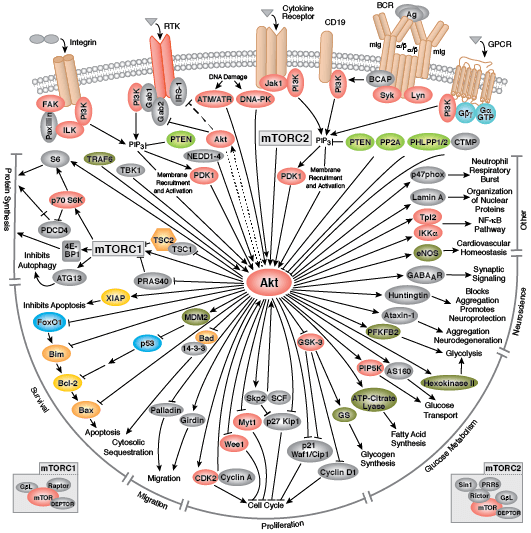
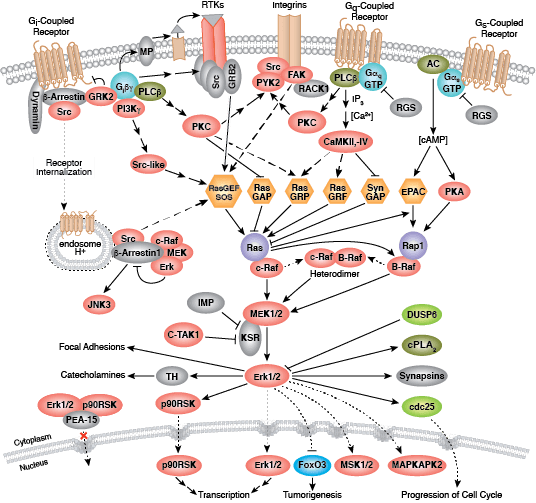
The serine/threonine kinase Akt/PKB exists as three isoforms in mammals. Akt1 has a wide tissue distribution, whereas Akt2 is found predominantly in muscle and fat cells and Akt3 is expressed in testes and brain. Akt regulates multiple biological processes including cell survival, proliferation, growth, and glycogen metabolism. Various growth factors, hormones, and cytokines activate Akt by binding their cognate receptor tyrosine kinase (RTK), cytokine receptor, or GPCR and triggering activation of the lipid kinase PI3K, which generates PIP3 at the plasma membrane. Akt binds PIP3 through its pleckstrin homology (PH) domain, resulting in translocation of Akt to the membrane. Akt is activated through a dual phosphorylation mechanism. PDK1, which is also brought to the membrane through its PH domain, phosphorylates Akt within its activation loop at Thr308. A second phosphorylation at Ser473 within the carboxy terminus is also required for activity and is carried out by the mTOR-rictor complex, mTORC2.
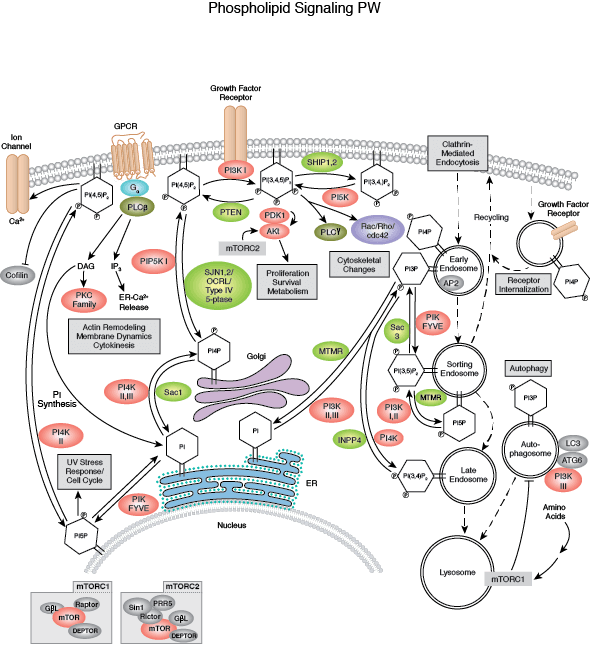
PTEN, a lipid phosphatase that catalyzes the dephosphorylation of PIP3, is a major negative regulator of Akt signaling. Loss of PTEN function has been implicated in many human cancers. Akt activity is also negatively regulated by the phosphatases PP2A and PHLPP, as well as by the chemical modulators wortmannin and LY294002, both of which are inhibitors of PI3K.
Activated Akt phosphorylates a large number of downstream substrates containing the consensus sequence RXRXXS/T. One of its primary functions is to promote cell growth and protein synthesis through regulation of the mTOR signaling pathway. Akt directly phosphorylates and activates mTOR, as well as inhibits the mTOR inhibitor proteins PRAS40 and tuberin (TSC2). Combined, these actions promote cell growth and G1 cell cycle progression through signaling via p70 S6 Kinase and inhibition of 4E-BP1.
GSK-3 is a primary target of Akt and inhibitory phosphorylation of GSK-3α (Ser21) or GSK-3β (Ser9) has numerous cellular effects such as promoting glycogen metabolism, cell cycle progression, regulation of wnt signaling, and formation of neurofibrillary tangles in Alzheimers disease. Akt promotes cell survival directly by its ability to phosphorylate and inactivate several pro-apoptotic targets, including Bad, Bim, Bax, and the forkhead (FoxO1/3a) transcription factors. Akt also plays an important role in metabolism and insulin signaling. Insulin receptor signaling through Akt promotes Glut4 translocation through activation of AS160 and TBC1D1, resulting in increased glucose uptake. Akt regulates glycolysis through phosphorylation of PFK and hexokinase, and plays a significant role in aerobic glycolysis of cancer cells, also known as the Warburg Effect.
Aberrant Akt signaling is the underlying defect found in several pathologies. Akt is one of the most frequently activated kinases in human cancer as constitutively active Akt can promote unregulated cell proliferation. Abnormalities in Akt2 signaling can result in diabetes due to defects in glucose homeostasis. Akt is also a key player in cardiovascular disease through its role in cardiac growth, angiogenesis, and hypertrophy.
References:
- Robey RB, Hay N (2009) Is Akt the "Warburg kinase"?-Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol. 19(1), 25–31.
- Zhang S, Yu D (2010) PI(3)king apart PTEN's role in cancer. Clin. Cancer Res. 16(17), 4325–30.
- Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12(1), 21–35.
- Zhang X, Tang N, Hadden TJ, Rishi AK (2011) Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta 1813(11), 1978–86.
- Kloet DE, Burgering BM (2011) The PKB/FOXO switch in aging and cancer. Biochim. Biophys. Acta 1813(11), 1926–37.
- Hers I, Vincent EE, Tavars JM (2011) Akt signalling in health and disease. Cell. Signal. 23(10), 1515–27.
- Wang H, Zhang Q, Wen Q, Zheng Y, Lazarovici P, Philip L, Jiang H, Lin J, Zheng W (2012) Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell. Signal. 24(1), 17–24.
- Dazert E, Hall MN (2011) mTOR signaling in disease. Curr. Opin. Cell Biol. 23(6), 744–55.
- Bayley JP, Devilee P (2012) The Warburg effect in 2012. Curr Opin Oncol 24(1), 62–7.