Cell Death and Disease
Investigating Apoptosis, Autophagy, and Necroptosis in Neurodegenerative Disease and Cancer
To support your ongoing neurodegeneration and oncology research, CST scientists are constantly developing and validating novel antibodies against important targets in relevant applications.
Neurodegenerative Disease
Neurodegenerative diseases, including Alzheimers disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD), are characterized by loss of neuronal function and cell death. Understanding the contribution of programmed cell death signaling pathways to disease progression remains a central goal of these fields.
Apoptosis
Apoptosis may be initiated via surface death receptors or mitochondrial dysfunction as a result of oxidative stress. Extracellular amyloid plaques and intracellular β-Amyloid can lead to activation of both presynaptic and postsynaptic apoptotic signaling. A number of proapoptotic hallmarks are elevated in neurodegenerative diseases.1
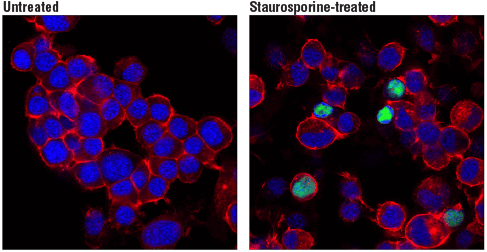
Cleaved PARP (Asp214) (D6X6X) Rabbit mAb (Rodent Specific) #94885: Confocal immunofluorescent analysis of Neuro-2a cells, untreated (left, negative) or treated with Staurosporine #9953 (1 μM, 3 hr; right, positive), using Cleaved PARP (Asp214) (D6X6X) Rabbit mAb (Rodent Specific) (green). Actin filaments were labeled with DyLight™ 554 Phalloidin #13054 (red). Blue pseudocolor = DRAQ5® #4084 (fluorescent DNA dye).
Autophagy
Mutations in the autophagy pathway, including SQSTM1/p62 family members, PTEN-induced putative kinase (PINK1), and Parkin, are associated with neurodegenerative diseases such as PD. Increases in LC3-positive microglia are observed in tissue from patients harboring AD-associated TREM2 mutations and TREM2 KO mice, suggesting that disruption of TREM2-dependent autophagy can contribute to AD etiology.2
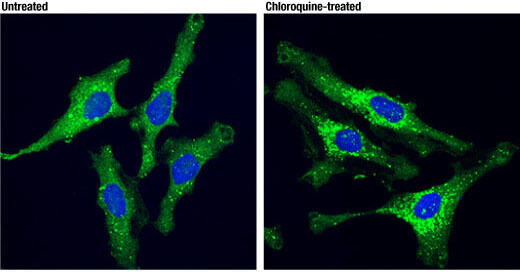
LC3B (E5Q2K) Mouse mAb #83506: Confocal IF analysis of MIA PaCa-2 (upper) and C2C12 (lower) cells, untreated (left), nutrient-starved with EBSS (2 hr; middle), or chloroquine treated (50 μM, 24 hr; right), using LC3B (E5Q2K) Mouse mAb (green) and β-Actin (D6A8) Rabbit mAb #8457 (red). Blue = Hoechst 33342 #4082 (fluorescent DNA dye).
Necroptosis
Necroptosis is a form of programmed cell death triggered by stress/inflammation and has been found to be activated in AD, multiple sclerosis (MS), and Amyotrophic Lateral Sclerosis (ALS). Activation and assembly of the necrosome complex containing receptor-interacting protein kinase 1 (RIPK1) and RIPK3 lead to phosphorylation and oligomerization of mixed-lineage kinase domain-like (MLKL), followed by lipid peroxidation, cation influx, and ultimately, cell death.3
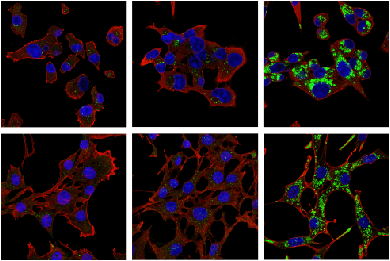
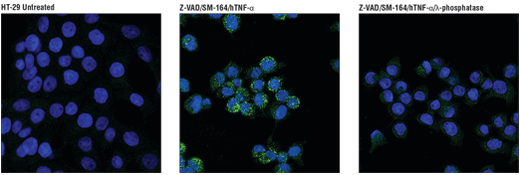
Phospho-RIP3 (Ser227) (D6W2T) Rabbit mAb #93654: Confocal IF analysis of HT-29 cells, untreated (left), pretreated with Z-VAD (20 μM, 30 min) followed by treatment with SM-164 (100 nM) and Human Tumor Necrosis Factor-α (hTNF-α) #8902 (20 ng/mL, 6 hr; center), or pre-treated with Z-VAD followed by treatment with SM-164 and hTNF-α and post-processed with λ-phosphatase (right), using #93654 (green). Blue pseudocolor = DRAQ5® #4084 (fluorescent DNA dye).
Oncology
The ability to evade programmed cell death is one of the hallmarks of cancer cells. Understanding mechanisms malignant cells use to escape this cellular function increases our comprehension about cancer progression and offers potential therapeutic targets.
Apoptosis
Dysregulated apoptosis drives tumorigenesis when there is a loss of balance between cell division and cell death. Reduced apoptosis increases tumor growth and can occur when there is an increase of anti- vs pro-apoptotic Bcl-2 family members, inactivation of tumor suppressors like p53, or upregulation of caspase-inhibiting proteins. Additionally, decreased expression or inactivating mutations in death receptor signaling pathway proteins, like CD95, can decrease apoptosis in malignant cells.4
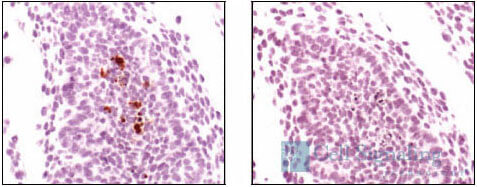
Phospho-p53 (Ser33) Antibody #2526: Immunohistochemical analysis of paraffin-embedded human breast carcinoma, using Phospho-p53 (Ser33) Antibody.
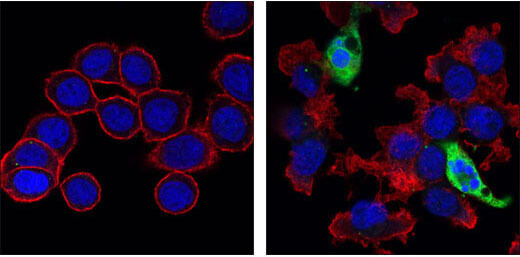
Cleaved Caspase-3 (Asp175) (5A1E) Rabbit mAb #9664: Confocal immunofluorescent images of HT-29 cells, untreated (left) or Staurosporine #9953 treated (right) labeled with Cleaved Caspase-3 (Asp175) (5A1E) Rabbit mAb #9664 (green). Actin filaments have been labeled with Alexa Fluor® 555 phalloidin (red). Blue pseudocolor = DRAQ5® #4084 (fluorescent DNA dye).
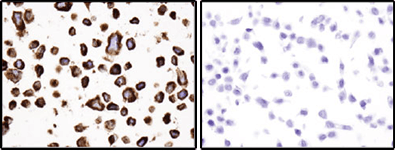
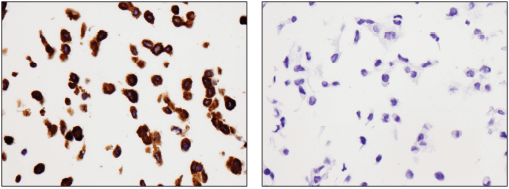
Bcl-2 (124) Mouse mAb #15071: Immunohistochemical analysis of paraffin-embedded RL (positive, left) and HT-29 (negative, right) cell pellets using Bcl-2 (124) Mouse mAb.
Autophagy
Autophagy is thought to have a dual tumorigenic role. Several autophagy-promoting genes have been found to function as tumor suppressors, including Beclin-1, UVRAG, SQSTM1/p62, and BNIP3. Inhibition of autophagic activity can promote tumor progression through loss of pathogen clearance and damaged organelles. In contrast, autophagy is thought to promote oncogenesis by enabling the survival of nutrient starved cancer cells. Drugs targeting autophagy are currently being evaluated as therapeutic strategies for cancer.5,6
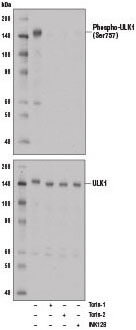
Phospho-ULK1 (Ser757) (D7O6U) Rabbit mAb #14202: Western blot analysis of extract from A172 cells, untreated (-) or treated with mTOR inhibitors, either Torin-1 (250 nM, 5 hr), Torin-2 (250 nM, 5 hr), or INK128 (250 nM, 5 hr) using Phospho-ULK1 (Ser757) (D7O6U) Rabbit mAb (upper) or ULK1 (D8H5) Rabbit mAb #8054 (lower).
SQSTM1/p62 (D6M5X) Rabbit mAb (Rodent Specific) #23214: Immunohistochemical analysis of paraffin-embedded MEF wild-type cell pellet (left, positive) or MEF SQSTM1/p62 KO cell pellet (right, negative) using SQSTM1/p62 (D6M5X) Rabbit mAb (Rodent Specific). MEF SQSTM1/p62 KO cells were kindly provided by Dr. Junying Yuan, Harvard Medical School, Boston MA.
Necroptosis
Necroptosis is thought to play multiple roles in cancer. It potentially facilitates metastasis by promoting inflammation of non transformed cells in the tumor microenvironment. Necrotic cells also provide antigens and inflammatory stimulation for the cross-priming required to activate anti-tumor immunity. Necroptosis mediators, like RIPK3, CYLD, and MLKL, are also commonly downregulated in various cancer types including, but not limited to, acute myeloid leukemia, breast carcinoma, and cervical carcinoma.7
Phospho-RIP3 (Ser227) (D6W2T) Rabbit mAb #93654: Confocal immunofluorescent analysis of HT-29 cells, untreated (left), pre treated with Z-VAD (20 μM, 30 min) followed by treatment with SM-164 (100 nM) and Human Tumor Necrosis Factor-α (hTNF-α) #8902 (20 ng/mL, 6 hr; center), or pre treated with Z-VAD followed by treatment with SM-164 and hTNF-α and post-processed with λ-phosphatase (right), using Phospho-RIPK3 (Ser227) (D6W2T) Rabbit mAb (green). Blue pseudocolor = DRAQ5® #4084 (fluorescent DNA dye).
References
- Radi E, et al. (2014) J Alzheimers Dis. 42, S125-52.
- Ulland TK, et al (2017) Cell. 170, 649-663.e13.
- Sun L, et al (2012) Cell. 148, 213-27.
- Wong RS, et al. (2011) J Exp Clin Cancer Res. 30(1), 87.
- Levine B, Yuan J. (2005) J Clin Invest. 115, 2679-2688.
- Galluzzi L, et al. (2015) EMBO J. 34(7), 856-880.
- Najafov A, et al. (2017) Trends Cancer. 3(4), 294-301.
Key Cell Death Markers
Apoptosis Markers
Autophagy Markers
| Atg12 |
| Atg5 |
| Atg7 |
| Phospho-Beclin-1 |
| Beclin-1 |
| BNIP3L/Nix |
| LC3A/B |
| Optineurin |
| SQSTM1/p62 |
| Phospho-Ubiquitin |
| ULK1 |
| Phospho-ULK1 |
Necroptosis Markers
| Phospho-MLKL |
| Phospho-RIP |
| RIP |
| Phospho-RIP3 |
| RIP3 |