Learn About the Fundamentals of Neuroscience
What Is Neuroscience?
Neuroscience is the study of the brain and nervous system, including the spinal cord, central nerves, and peripheral nerves. Some of the most exciting questions in cutting edge scientific research center around how the brain is formed and organized and how it functions. In addition, treatment and prevention of neurological disorders, such as chronic pain and psychiatric, neurodevelopmental, and neurodegenerative disorders, are at the forefront of modern medicine today. We will predominantly focus on the brain, but many principles discussed below can be applied to the spinal cord and, in some cases, even to central and peripheral nerves.
Neurodevelopment
Neurodevelopment focuses on the molecular and cellular events that govern the development of the brain. Central nervous system (CNS) development (ie, brain and spinal cord) begins with the establishment of the neural crest, which folds to form the neural tube as early as gestational week (GW) 3 in humans. The fusion of the neural tube leads to formation of the neuroepithelium, a single-cell layer composed of multipotent stem cells. Various graded signals generate distinctive neuroepithelial niches within the dorsal, ventral, anterior, and posterior sections of the neural tube. These unique neuroepithelial progenitor cells (NEPs) contribute to the regionalization of the neural tube and over time proliferate, migrate, and differentiate, leading eventually to the formation of unique structures within the brain and spinal cord. NEP position along the anteroposterior and dorsoventral axes of the neural tube defines their identity and imbues their progeny with similar properties through exposure to regional patterning signals.
By GW5, neuron production (ie, neurogenesis) begins, peaking between GW8 and GW25 and largely concluding by midgestation. Embryonic neurogenesis takes place in specialized regions along the ventricle, where cerebrospinal fluid circulates. These specialized areas of rapid and robust proliferation of NEPs are known as germinal zones. A governing principal of neural development is that the multipotency of neural progenitors is progressively restricted over time and with rounds of cell division. Therefore, cells only become fate-restricted late in their developmental history. As such, by GW8, once multiple rounds of cell division have occurred, neural precursors begin to differentiate into postmitotic neurons. These fate-restricted postmitotic neurons migrate away from their birthplace near the ventricle to populate the cortical plate, a transient structure that will give rise to future layers of the part of the brain known as the neocortex. Neuronal migration peaks between GW12 and GW20 and is completed by GW29. Thus, neocortical lamination, or subdivision into 6 distinct layers, is encoded early during gestation. Once neuronal migration begins, layer formation proceeds in an inside-out fashion with deeper layers (ie, those closer to the ventricle) forming prior to superficial layers (ie, those closer to the pia).
Similar to neurogenesis, gliogenesis-or the generation of (macro)glial cells within the CNS-occurs during embryonic development. The peak of gliogenesis of astrocytes and oligodendrocytes from NEPs takes place during gestation between GW20 and GW40, after the peak of neurogenesis has passed. Generation and maturation of the microglia occur over time, with an initial colonization at GW9 that continues to expand through GW22. Differentiated microglia can be observed at GW35.
Another process occurring during embryonic development is the formation of synaptic connections among neurons. The earliest synapses are observed around GW8 in the spinal cord and GW9-10 in the brain. After the formation of the cortical plate in the brain, cortical synaptogenesis gradually increases, peaking at GW28. Postmortem neuroanatomical studies coupled with magnetic resonance imaging (MRI) have shown that neurons at this point connect in a rudimentary network that drives the generation of major fiber pathways by the end of gestation. Thus, the basic framework for the brain connectome is generally set by the end of the prenatal period. However, synaptic connections exhibit dynamic changes throughout life in response to experience and environmental changes.
While neocortical expansion, myelination, and synapse formation are achieved during gestation, brain growth is still robust after birth. In fact, only by age 6 does the brain finally reach 90% of its adult volume, having increased in size 4-fold during adolescence. Growth of specialized cortical regions continues well beyond this age and into adulthood. Continual reorganization of synaptic connections postnatally is the underlying principal of neural plasticity; however; it is clear that the framework established during embryonic development sets the stage for neural outcomes and function throughout postnatal life.
Neural and Glial Cell Types
As mentioned above, neurons and macroglia (ie, astrocytes and oligodendrocytes) differentiate from common progenitor cell populations. However, microglia develop from macrophages that arise outside the CNS but are seeded into the brain as a result of normal circulation and through the ventricular system.
Neurons have long been thought to be the “principal” cells of the CNS, while glia retained a more supportive role. However, many recent studies have demonstrated that both neurons and glia play major roles in modulation of brain function and can thus contribute to neurological diseases in profound ways. Typically, cell types in the brain can be identified based on their morphology as well as expression of unique proteins. Below, we will discuss these major cell populations in more detail.
Neurons
Neurons are the specialized cells of the CNS that receive and transmit electrical impulses due to their ability to process electrochemical signals. These cells typically exhibit a complex morphology consisting of a cell body, referred to as the soma, branched processes for the intake of information, referred to as dendrites, and a long and slender process that may or may not travel long distances to send out information to other neurons, known as an axon.
Neurons can be subdivided into groups based on the type of neurotransmitters they express, their polarity, morphology, anatomical localization, and the direction in which they transmit information:
- Type of neurotransmission: neurons can either be excitatory or inhibitory based on whether the neurotransmitters that they release depolarize (ie, make more positive) or hyperpolarize (ie, make more negative) the target neurons that they interact with.
- Polarity: the orientation of the axons and dendrites in relation to the soma can be used to classify excitatory neurons into 4 major groups:
- Unipolar: contains only 1 process; only common in insects
- Bipolar: contains the 2 prototypical extensions-1 axon and 1 basal dendrite that eventually branches into dendritic processes
- Multipolar: contains 1 axon and many dendrites
- Pseudounipolar: contains 1 extension that serves as both an axon and dendrite
- Morphology: the shape and size of the soma, as well as the polarity of the processes, can be used to further substratify excitatory neurons.
- Anatomical location: the location where the neuron resides within the CNS confers specific characteristics onto the neurons and thus adds another important property to consider when classifying a particular neuron.
- Information flow: because of their specialized processes, neurons naturally instill a directionality in the way they transmit information:
- Afferent neurons: neurons that carry information from an organ or specific tissue to the CNS
- Efferent neurons: neurons that carry information to an organ or specific tissue from the CNS
- Interneurons: neurons that transmit information from one neuron to another within the CNS
In essence, in combination, these characteristics can be used to characterize and categorize any neuron in the CNS and can give tangible information about the function and connectivity of that particular neuron. Importantly, areas of the CNS where neuronal soma can be found are referred to as “gray matter,” while areas of the CNS where axons can be found are called “white matter.” More information about white matter will be discussed in the Oligodendrocytes and Schwann Cells section.
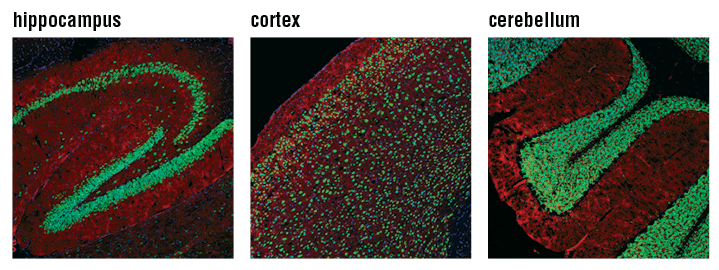
Confocal immunofluorescent analysis of mouse hippocampus (left), cortex (middle), and cerebellum (right) using NeuN (D4G4O) XP® Rabbit mAb #24307 (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054 (red). Blue pseudocolor = DRAQ5 #4084 (fluorescent DNA dye).
Microglia and Astrocytes
Astrocytes and microglia are both immune-related cells. Although once thought to only contribute to immune responses to noxious stimuli in the brain, new roles have emerged for both astrocytes and microglia in modulating neuronal activation and synaptic communication.
Microglia are the resident immune cells of the CNS. They arise from the myeloid lineage and infiltrate the brain during early embryogenesis, where they rapidly proliferate and mature, populating both white and gray matter. During development, and in areas of adult neurogenesis, microglia serve to regulate the assembly of complex neural networks. They eliminate redundant neurons, synapses, and dendrites through pruning. They also contain certain neurotransmitter receptors and can aid in synaptic plasticity in response to neurotransmitter release from neurons. In addition, they can prevent excitotoxicity when excessive amounts of excitatory neurotransmitters are released by neurons.
Microglia also play major roles in disease states, in particular in neurodegenerative disease where protein aggregates play a major role. These include Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS). In healthy states, microglia exist in a quiescent pool. In the event of infection or acute trauma to the brain, microglia become activated and mount an inflammatory response through the release of pro-inflammatory molecules. In certain cases, when cellular debris is resent, microglia can become phagocytic to remove this debris. This is typically an acute response that resolves over time. However, in disease states, chronic inflammation in response to aggregated protein species or other long-term noxious stimuli generates a chronic pro-inflammatory response known as gliosis, which is extremely detrimental to the health of neurons, astrocytes, and oligodendrocytes. Thus, while microglia aid in protecting the brain when acute adverse events take place, prolonged microglial activation as a result of a disease state contributes to the progression of the disease.
Unlike microglia, astrocytes are born in the CNS. They are also thought to contribute to brain immunity because they serve to support the function of the endothelial cells that make up the blood brain barrier, and they become reactive in response to a noxious stimulus. Astrocytes contribute to scar formation and repair of the CNS after injury. On the other hand, strong interplay between neurons and astrocytes further serves to diversify astrocytic function in the CNS. For example, astrocytes can buffer the extracellular concertation of certain ions that could impact neuronal function and can respond to certain neurotransmitters as well release neurotransmitters (ie, gliotransmitters) themselves. In addition, neurons and astrocytes are strongly metabolically linked. Astrocytes provide nutritive support to neurons as well as aid in the biosynthesis of a principle neurotransmitter through the release of a critical intermediary. Like microglia, astrocytes play a role in both synaptogenesis and synaptic maintenance. Also similar to microglia, chronic disease states can lead to prolonged activation of astrocytes and astrogliosis. As such, astrocyte-driven lesions are characteristic of many neurodegenerative diseases, including AD and Huntington’s disease (HD).
Crosstalk between astrocytes and microglia further leads to modulation, exacerbation, or extinction of their respective functions in healthy and disease states, adding to their impact on overall brain function.
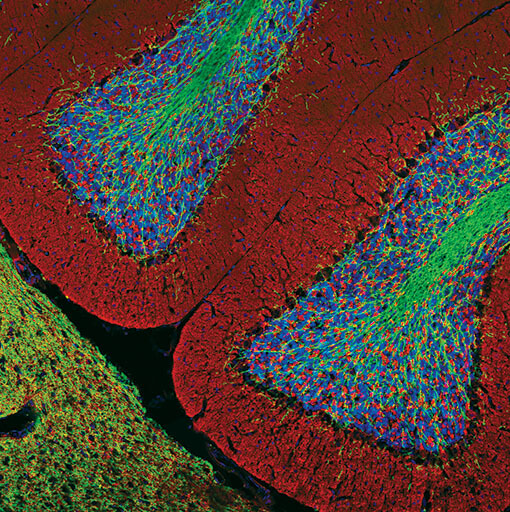
Confocal immunofluorescent analysis of normal rat cerebellum using GFAP (GA5) Mouse mAb (Alexa Fluor® 555 Conjugate) #3656 (red) and Neurofilament-L (C28E10) Rabbit mAb #2837 (green). Blue pseudocolor = DRAQ5 #4084 (fluorescent DNA dye).
Oligodendrocytes and Schwann Cells
Oligodendrocytes (OLs) are the myelin-producing cells of the CNS. As a general rule, oligodendrogenesis always follows neurogenesis and typically arises from the same brain and spinal cord regions as neurons. Mature OLs produce a lipid-rich membrane, known as myelin, to insulate axons and increase the conduction velocity of electrical signals among neurons. The process of myelination begins at GW12 in the spinal cord and GW14 in the brain, becoming robust only during the first postnatal year, and continuing into the third decade of life. Schwann cells are equivalent to OLs but reside in the peripheral nervous system.
Myelination is a dynamic process that is finely tuned to augment neuronal communication. A regenerating pool of oligodendrocyte precursor cells remains in the brain throughout life to ensure the generation of new OLs as needed. Oligodendrogenesis can be triggered due to the loss of myelin in response to an injury or when myelin remodeling is needed following a change in the neuronal network. However, despite the presence of these precursors, age-related decline in myelination has been linked to the onset of dementia and decreased cognitive function. In addition, loss of myelin had been observed in neurodegenerative diseases such as AD and multiple sclerosis (MS).
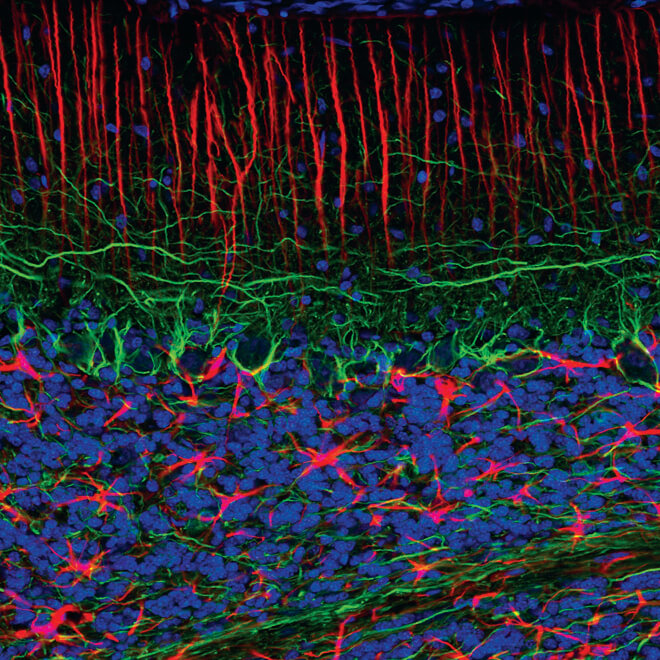
Confocal immunofluorescent analysis of rat brain using Myelin Basic Protein (D8X4Q) XP® Rabbit mAb #78896 (green) and Synaptophysin (7H12) Mouse mAb (IF Formulated) #9020 (red). Blue pseudocolor = DRAQ5 #4084 (fluorescent DNA dye).
Nervous System Function
The interaction of neurons and glia modulates nervous system function. Below, we discuss the intracellular events that mediate electrical signal propagation in neurons as well as the input from extraneural systems, both of which also provide modulation of CNS function.
Synaptic Activity
As discussed above, neurons are specialized cells that can process electrochemical signals. Neurons contain processes that allow for signal input and output (ie, dendrites and axons, respectively). The neuron originating the signal is known as the presynaptic cell, while the neuron receiving the signal is known as the postsynaptic cell, and the extracellular space between the 2 is known as the synapse.
Ligand and voltage-gated ion channels in the presynaptic cell allow for the influx of ions that can impact the intracellular voltage, also referred to as the membrane potential, of the neuron. Typically, the resting membrane potential of a neuron is -50 to -70 mV. The rapid influx of cations into the cell depolarizes the presynaptic neurons, and when it reaches a particular threshold, the neuron fires an action potential. This is an all-or-nothing response to the change in potential that propagates through the entire axon until it reaches the presynaptic terminals where neurotransmitter vesicles are primed and ready for calcium-mediated docking to the presynaptic membrane. Upon calcium influx into the presynaptic terminal in response to the resident voltage-gated calcium channels, synaptic vesicles containing neurotransmitters bind the membrane and allow for extracellular release of these molecules. The neurotransmitters then traverse the synapse and bind to postsynaptic receptors on subportions of the dendrites of postsynaptic cell, known as spines.
Depending on the neurotransmitters that are released and the receptors that they bind, the postsynaptic cell can be inhibited or excited due to the influx of negative or positive ions, respectively. If the positive ions depolarize the postsynaptic cell, then it fires an action potential. However, if the ions hyperpolarize the postsynaptic cells or fail to depolarize this cell, no action potential is fired, and the signal is not propagated. In the presynaptic cell, once the action potential passes, the cell repolarizes through the action of ion channels and ATP-dependent transporters that normalize the intracellular environment. A brief hyperpolarization stage disallows the firing of a new action potential; this is known as a refractory period. Once this period passes, the cell can then be depolarized once again. Rapid firing of the presynaptic cell impacts the receptor pool on the postsynaptic cell leading to remodeling of the interaction. This is a form of synaptic plasticity. Other forms include synaptic pruning and modulation through the actions of glia.
Circadian Rhythms
Circadian rhythm is a biological process that confers a periodicity to other biological functions in an organism over a 24-hour cycle. One major example of a circadian behavior is the sleep-wake cycle. This cycle is entrained by light signals that are processed by the brain. In the brain, a small group of neurons in the hypothalamus function as a master circadian pacemaker controlling the timing of the sleep-wake cycle, which is an important indirect regulator of other behaviors, such as metabolism and memory consolidation. Thus, the brain serves as the seed of circadian regulation in a way that feeds back to support the function of the CNS.
Sensory and Motor Systems
Sensory and motor systems rely heavily on the action of peripheral nerves. Stimuli initiating a sensation in the skin travel through a network of nerves that make synapses in both the spinal cord and the brain. The areas of the brain that encode sensory and motor functions are organized in a topographic map that corresponds to specific areas of the body; this is known as the homunculus. The route of the nerves from the periphery to the brain is circumscribed and, therefore, precise anatomical mapping can be used to determine where a lesion might be if sensory or motor function is affected.
Endocrine System
The endocrine system and the nervous system are highly intertwined. Hormones regulate many aspects of brain function as well as modulate many processes that directly or indirectly affect the brain, such as feeding and metabolism, neurotransmitter synthesis and release, vascular function and blood flow, and even child birth. Hormones can impact certain neuronal circuits, further reinforcing behavior or contributing to its extinction. In addition, sexual differentiation of the brain by estrogen is a prominent factor during brain development, and changes in estrogen levels in postmenopausal women may relate to age-related changes in brain function. Thus, the interconnectedness of the endocrine and nervous systems is another level of modulation that affects overall function.
Disorders of the Nervous System
Disorders of the nervous system span multiple categories that are discussed briefly below.
Neurodegeneration
The term “neurodegeneration” generally refers to disorders of the CNS that lead to loss of neurons or glia. Neurodegeneration can be related to age-related diseases (AD, PD, dementia), autoimmune attacks on the nervous system (MS), genetic mutations that affect the heath of CNS cells (HD, early onset AD/PD, ALS), or even due to mechanical insults to the brain (chronic traumatic encephalitis). Several of these disease will be discussed below.
5.1a Alzheimer’s Disease (AD)
Alzheimer’s disease (AD) affects approximately 50 million people world-wide, and this number is expected to steadily increase over the next decade, creating a growing global public health burden.
The hallmarks of AD are intracellular and extracellular protein aggregates that result in what is clinically known as plaques and tangles. Along with the accumulation of these pathological protein species, widespread neuronal loss in multiple brain regions takes place, leading to incremental deterioration in cognitive function. As mentioned above, not only are neurons impacted in AD, glia are also heavily implicated. However, whether they are primary effectors or secondary correlates of AD is still unclear.
Although AD is generally thought of as an aging-related disorder, mutations in certain genes can lead to an early onset version of AD. Investigating these specific mutations has been used as a tool to identify and characterize major molecular players that likely contribute to the pathogenesis of both types of AD. Targeting these genes and their protein products therapeutically along with developing vaccines against the noxious protein aggregates are 2 of the most widely used strategies developed to combat AD. To date, there is no cure for AD.
5.1b Parkinson’s Disease (PD)
Similar to AD, Parkinson’s disease (PD) is both an age-related and a genetically based disease. Protein aggregation is also a hallmark of PD, yet the etiology of these aggregates is more diverse than in AD. This is because familial or early onset PD can arise from far more genetic mutations than those in AD, making therapeutic development in PD more complex. PD first manifests as a motor disease, but progressive neurodegeneration eventually leads to cognitive decline.
5.1c Multiple Sclerosis (MS)
In multiple sclerosis (MS), the immune system attacks the myelin sheaths that surround neuronal axons in the brain and spinal cord, leading to poor neuronal communication and deterioration of the axons themselves. MS leads to changes in locomotive ability and can have more widespread effects on vision, pain processing, speech, and mood. The exact causes of MS are unknown, but women are twice as likely to develop the disease, and race and environmental factors are risk factors.
5.1d Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS) specifically affects neurons in the brain and spinal cord that innervate muscle. These are known as motor neurons. There is progressive degeneration and loss in motor neurons that leads to the inability of the CNS to control muscle movement. This leads to the loss in the ability to speak, move, eat, and eventually breathe. Ninety percent to 95% of cases of ALS are sporadic, while 5% to 10% of cases of ALS are familial. Although the specific causes of ALS are unknown, specific mutations have been linked to the development of the disease, along with changes in neurotransmission, immune response, and protein handling.
Neurodevelopmental Disorders
Unlike neurodegenerative disorders, neurodevelopmental disorders arise during CNS development. These can be genetically based (Autism, Down syndrome, and Fragile X syndrome), environmentally triggered (fetal alcohol syndrome), or completely idiopathic (autism).
5.2a Autism
Autism is a neurodevelopmental disorder because it can arise from changes in CNS development during gestation or during early adolescence. Autism can occur as a result of genetic mutations, environmental factors, certain infections, or hypoxia during birth. Autism is a highly complex disorder and manifests differently among affected individuals. In general, autism is characterized by social-interaction and communication difficulties, along with repetitive behaviors. These general hallmarks can vary from mild to severe and can be accompanied by a constellation of other symptoms, such as anxiety, hyperactivity, sensory processing problems, seizures, sleep disorders, gastrointestinal diseases, and mood changes.
5.2b Down Syndrome (DS)
Down syndrome (DS) is the most common autosomal aneuploidy. There are approximately 400,000 people living with DS in the United States, with 5,300 babies with DS born annually. DS is also known as trisomy 21 and is purely genetically based. It is considered a neurodevelopmental disorder because people with DS have altered development and growth of the CNS leading to changes in cognition. Because neurons and glia divide and mature at slower rates in people with DS, widespread changes in neuronal communication and function are seen over the lifespan of people with DS. In addition, due to triplication in one specific AD-related gene that is on chromosome 21, people with DS are more susceptible to also having AD.
Psychiatric Disorders
Psychiatric disorders typically arise when there is an imbalance in neurotransmission. One example of such a disorder is schizophrenia. Schizophrenia is a chronic mental disorder that can be hereditary or environmental and is characterized by changes in neuronal communication that may arise from altered neurotransmitter levels or from changes in brain development during gestation. In addition, endocrine changes that occur during puberty can trigger the onset of schizophrenia, but the exact etiology is unknown. Since the root cause of schizophrenia is still unknown, treatments target symptoms and can range from antipsychotics to psychotherapy.
Pain Disorders
Pain disorders are characterized as chronic pain that is severe enough to interfere with day-to-day function. While some pain disorders are thought to be associated with psychological factors, some arise from changes in the health of peripheral nerves (ie, peripheral neuropathy) and/or the CNS. For example, peripheral neuropathy can result from injuries, genetic causes, exposure to toxins, or metabolic diseases such as diabetes. Another pain disorder is fibromyalgia, which is characterized as widespread musculoskeletal pain that causes repeated nerve stimulations, inducing synaptic plasticity, thereby changing neurotransmission and processing of painful stimuli by the brain.
How Is Neuroscience Studied?
Neuroscience is typically studied using model systems, postmortem human tissue, or imaging technology in patients. Model systems as well as specific tissue-based techniques will be discussed below.
Model Systems
The most classical model system for the study of the neuroscience was the squid giant axon. This system was attractive because the axon is easy to isolate and to visualize without a microscope. Classical studies about action potential propagation and neurotransmitter release were conducted in the squid giant axon. More contemporary model organisms range from the fruit fly to worms, zebrafish, mice, rats, ferrets, and nonhuman primates. Fruit flies, worms, and rodents offer a unique system in which genetic manipulations can be made in order to isolate important aspects of a neural circuit or a specific protein that may be essential for function. In addition, mutations that are known to give rise to certain diseases can be induced in those organisms, and many aspects of CNS function, including physiology, molecular biology, anatomy, and behavior, can be studied. In addition, neuroscience can also be modeled using cells derived from any of these organisms, and from humans, both healthy and diseased.
Techniques
Neuroscience techniques range from basic cellular and molecular techniques, to electrophysiological assessments, to imaging-based techniques that utilize antibody-based technology to visualize the system in situ.
In particular, in situ histological staining is a highly flexible, robust, and informative method that can be used to identify proteins of interest and their subcellular localization and to visualize how these proteins interact. Individual neuronal and glial populations can be isolated and characterized, along with their axons, dendrites, receptors, and neurotransmitter content. In addition, proteins that are altered in diseases states can be selectively marked. Cell Signaling Technology has a large repertoire of neuroscience tools that can be used for this in situ detection as well as other means of identifying proteins of interest.