N-Terminal AcetylScan® Proteomics
Protein N-terminal acetylation is a common post-translational modification (PTM) that typically occurs on newly translated proteins, but can also occur independently of new protein synthesis. N-terminal acetylation is a conserved modification from bacteria to higher eukaryotes. A group of enzymes, the N-terminal acetyltransferases (NATs) mediate this modification, with some NAT family members modifying nascent polypeptides on the initiator methionine, while other NATs acetylate the N-terminal residue post-excision of the initiator methionine. N-terminal acetylation regulates such processes as protein localization, protein-protein interaction, and protein stability. NATs have also been implicated in cancer, neurodegenerative disease, and other human hereditary lethal conditions.
N-AcetylScan® technology for N-terminal acetylation proteomics uses proprietary N-terminal acetyl (N-AC) antibodies to enrich N-acetyl-containing peptides from protease digested samples. N-acetylation antibodies from CST are rigorously tested, including ELISA, western blot, and PTMScan® testing to confirm specificity and sensitivity. This reagent is optimally designed and formulated to recognize only N-terminally acetylated peptides.
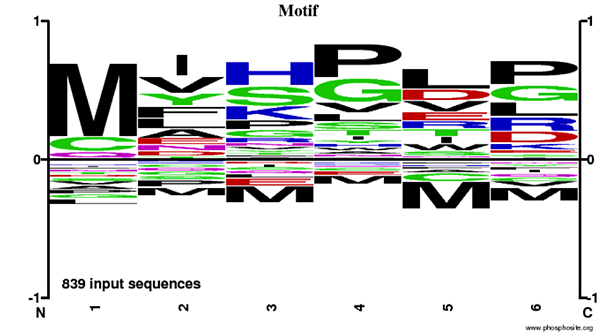
The Motif Logo was generated from an N-Terminal AcetylScan® LC-MS/MS experiment using 839 nonredundant tryptic peptides derived from mouse liver, brain, and embryo tissues immunoprecipitated with PTMScan® N-Terminal Acetyl Motif Immunoaffinity Beads. The logo represents the relative frequency of amino acids in each position starting from the N-terminal residue of each peptide within this data set.