Mixed Lineage Leukemia (MLL)
Mixed lineage leukemia is characterized by chromosomal translocations of the mixed lineage leukemia (MLL) gene to form fusion proteins with partner proteins. Many of these MLL-fusion partners belong to the super-elongation complex involved in regulating transcriptional elongation. MLL-fusions can create oncogenic transcription factors that cause aberrant transcriptional regulation of oncogenes or tumor suppressors to initiate oncogenesis.
Start with these targets
MLL1
MLL1 is processed into two polypeptides, both which are subunits of the wild-type MLL complex. The amino terminus of MLL1 is what is included in the fusion proteins that cause leukemogenesis. We recommend using a carboxy-terminal specific antibody for detecting wild-type MLL1 and an amino-terminal specific antibody for detecting MLL-fusion proteins.
Products
MLL1 (D6G8N) Rabbit mAb (Carboxy-terminal Antigen) #14197 – WB, IP, IF
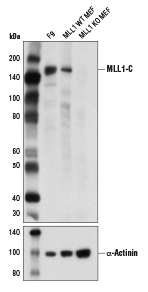
Western blot analysis of extracts from MLL1 wild-type (WT) and knockout (KO) mouse embryonic fibroblasts (MEF) using MLL1 (D6G8N) Rabbit mAb (Carboxy-terminal Antigen) (upper) and α-Actinin (D6F6) XP® Rabbit mAb #6487 (lower). MLL1 WT and KO MEF were kindly provided by Dr. Ali Shilatifard of Northwestern University.
MLL1 (D2M7U) Rabbit mAb (Amino-terminal Antigen) #14689 – WB, IP
MLL1 (E9W9Z) Rabbit mAb (Carboxy-terminal Antigen) #34907 – WB, IP, ChIP
MLLT1/ENL (D9M4B) Rabbit mAb #14893 – WB, ChIP, C&R, C&T
Menin
Menin is an adaptor protein and a tumor-suppressor encoded by the multiple endocrine neoplasia 1 (MEN1) gene. Menin also interacts with the carboxy-terminus of MLL1 and functions as a critical cofactor for MLL1 and MLL fusion proteins.
Products
Menin (E5P1R) Rabbit mAb #19893 – WB, IP, IHC, ChIP
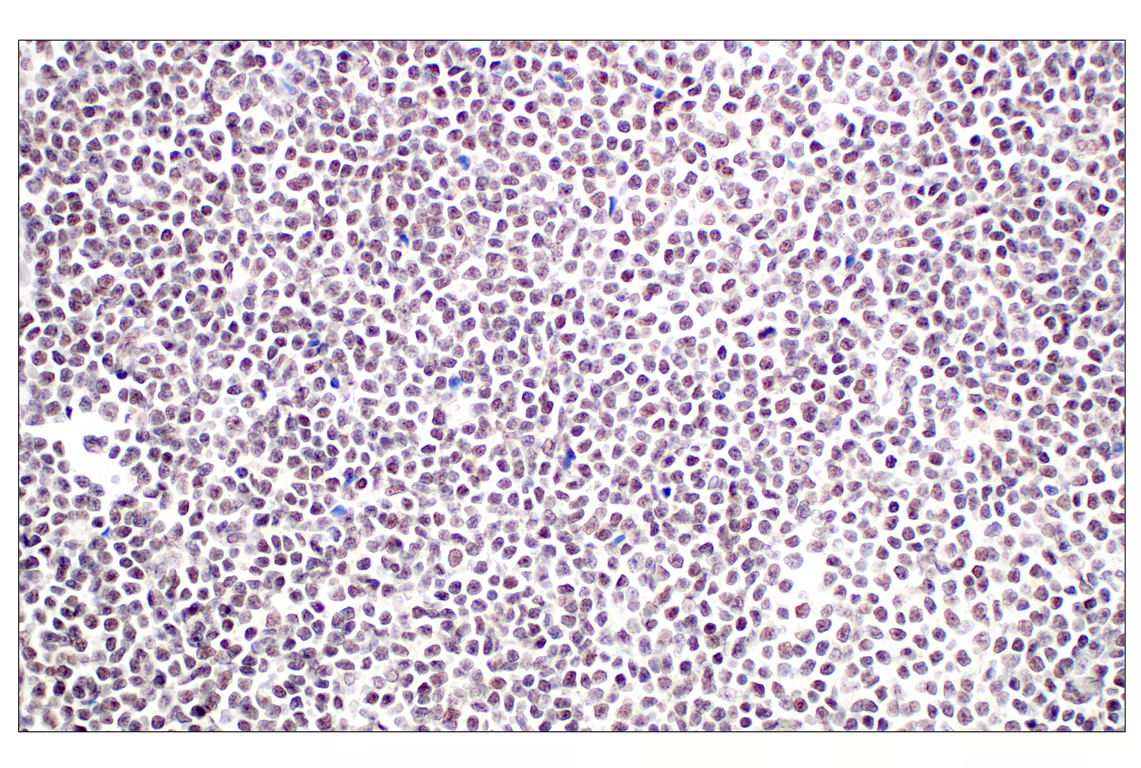
Immunohistochemical analysis of paraffin-embedded human B-cell non-Hodgkin lymphoma using Menin (E5P1R) Rabbit mAb.
Menin (D45B1) XP® Rabbit mAb #6891 – WB, IF
DOT1L
DOT1L interacts with MLL translocation partners in the super-elongation complex and is required for MLL-driven leukemiogenesis. DOT1L is thought to drive gene expression through the methylation of histone H3 at Lys79.
Products
DOT1L (D1W4Z) Rabbit mAb #77087 – WB, IP, ChIP
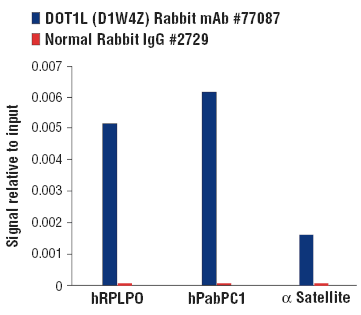
Chromatin immunoprecipitations were performed with cross-linked chromatin from 293T cells and either DOT1L (D1W4Z) Rabbit mAb or Normal Rabbit IgG #2729 using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. The enriched DNA was quantified by real-time PCR using SimpleChIP Human PabPC1 Exon 1 Primers #77410, Human RPLP0 Exon 2 Primers, and SimpleChIP Human α Satellite Repeat Primers #4486. The amount of immunoprecipitated DNA in each sample is represented as signal relative to the total amount of input chromatin, which is equivalent to one.
p300 and CBP
p300 and CBP are additional MLL-fusion proteins that can drive gene expression through histone acetylation.
Products
p300 (D8Z4E) Rabbit mAb #86377 – WB, IP, IF, IHC
p300 (D2X6N) Rabbit mAb #54062 – WB, IP, ChIP, ChIP-seq
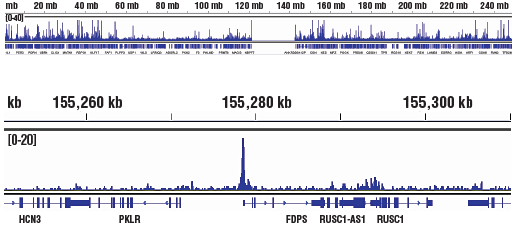
Chromatin immunoprecipitations were performed with cross-linked chromatin from K-562 cells and p300 (D2X6N) Rabbit mAb using SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) #9005. DNA Libraries were prepared from 5 ng enriched ChIP DNA using DNA Library Prep Kit for Illumina (ChIP-seq, CUT&RUN) #56795, and sequenced on the Illumina NextSeq. The figure shows binding across FDPS gene.
CBP (D6C5) Rabbit mAb #7389 – WB, IP, IF, ChIP, ChIP-seq
Histone Marks
Changes in levels of these marks as detected by ChIP/ChIP-seq may indicate altered recruitment of MLL1, DOT1L, and CBP/p300 to target genes.
Products
Tri-Methyl-Histone H3 (Lys4) (C42D8) Rabbit mAb #9751 – WB, IF, F, IHC, ChIP, ChIP-seq
Di-Methyl-Histone H3 (Lys79) (D15E8) XP® Rabbit mAb #5427 – WB, IF, F, IHC, ChIP
Tri-Methyl-Histone H3 (Lys79) (E8B3M) Rabbit mAb #74073 – WB, IP, IF, F, IHC, ChIP
Acetyl-Histone H3 (Lys9) (C5B11) Rabbit mAb #9649 – WB, IP, IF, F, IHC, ChIP, ChIP-seq