Immunohistochemistry (IHC) Troubleshooting Guide & the Importance of Using Controls
Expediting your time to results is critical. In order to save you as much time as possible, CST rigorously tests and develops a product-specific protocol, including optimal conditions for use of each antibody, validated for IHC. The conditions are worked out in advance by testing multiple methods of antigen retrieval and immunostaining, as well as developing companion reagents to enhance antigen detection and improve the efficiency of IHC protocols.
If you do need to perform some troubleshooting, suboptimal IHC staining is frequently resolved by adjusting relatively few variables. Adjustments to key steps within the protocol, such as antigen retrieval, can often resolve these common issues.
Immunohistochemistry Tips & Techniques
Immunohistochemistry Protocols
Potential Problem: Little to No Staining
Negative Staining
A complete lack of staining in your immunohistochemistry (IHC) experiment may indicate an issue with the antibody or protocol. Ensure your antibody is validated for the recommended application and employ a high-expressing positive control, such as paraffin-embedded cell pellets, to ensure that the antibody and procedure are working as expected.
Phospho-specific antibodies in particular, or any antibody directed against a rarely expressed protein, may not stain 100% of the cases of a given indication. It is also possible that the sample is truly negative.
Sample Storage or Dry Tissue Sections
Slides for IHC may lose signal over time in storage. The storage process can be variable and depends on the protein target. The effect of slide storage on staining has not been established for every protein; therefore, it is best practice that slides are freshly cut before use. If slides must be stored, do so at 4°C; we do not recommend baking slides before storage. It is vital that the tissue sections remain covered in liquid throughout the staining procedure.
Slide Preparation
Inadequate deparaffinization may cause spotty, uneven background staining in your IHC experiment. If this is the case, we recommend repeating the experiment with new tissue sections using fresh xylene.
Antigen Unmasking/Retrieval Buffer
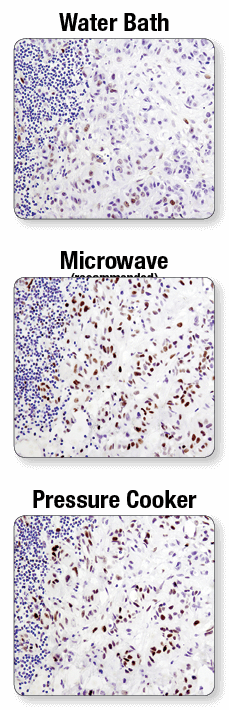
Fixed tissue sections used for IHC have chemical crosslinks between proteins that, depending on the tissue and antigen target, may prevent antibody access or mask antigen targets. Antigen unmasking protocols may utilize a hot water bath, microwave, or pressure cooker. Antigen unmasking protocols utilizing a water bath are not recommended. Antigen unmasking performed with a microwave is preferred, though staining of particular tissues or antigen targets may require the use of a pressure cooker.
Staining of particular tissues or antigen targets may require an optimized unmasking buffer. CST offers optimized protocols with recommended antigen unmasking buffers on each product data sheet. We also recommend to always prepare fresh 1X solutions daily.
To find product-specific protocols for all of our IHC-validated antibodies, start here.
A microwave oven is recommended for antigen retrieval. IHC analysis of paraffin-embedded human lung carcinoma using Phospho-Stat3 (Tyr705) (D3A7) XP® Rabbit mAb #9145 after antigen retrieval using a water bath (top), microwave oven (middle), or pressure cooker (bottom). A clear difference in performance is seen when using a microwave as compared with a water bath. For some antibodies, using a pressure cooker may enhance signals beyond those obtained with a microwave.
A microwave oven is recommended for antigen retrieval. IHC analysis of paraffin-embedded human lung carcinoma using Phospho-Stat3 (Tyr705) (D3A7) XP® Rabbit mAb #9145 after antigen retrieval using a water bath (top), microwave oven (middle), or pressure cooker (bottom). A clear difference in performance is seen when using a microwave as compared with a water bath. For some antibodies, using a pressure cooker may enhance signals beyond those obtained with a microwave.
Antibody Dilution/Diluent
CST offers optimized protocols with recommended dilutions and diluent for each IHC-validated antibody product. Titration of the antibody may be required if a reagent other than the one recommended is used.
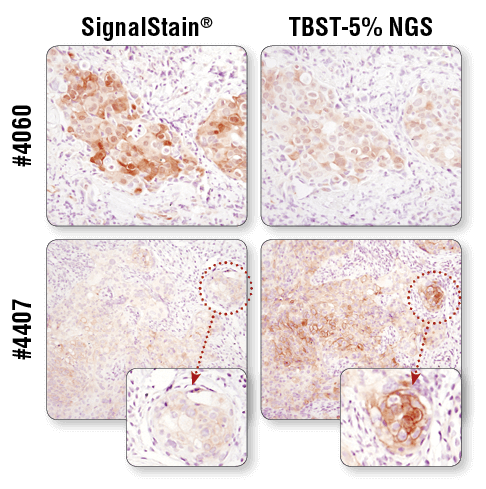
For optimal results, always use the recommended primary antibody diluent, as indicated on the product datasheet. IHC analysis of paraffin-embedded human breast carcinoma (top) and HCC827 xenograft (bottom) using Phospho-Akt (Ser473) (D9E) XP® Rabbit mAb #4060 or Phospho-EGF Receptor (Tyr1173) (53A5) Rabbit mAb #4407 after dilution in either SignalStain® Antibody Diluent (left) or TBST/5% NGS (right). As shown, a superior signal is achieved when #4060 is diluted in SignalStain® Antibody Diluent as compared with TBST/5% NGS. In contrast, #4407 performs better when diluted in TBST/5% NGS. Always check the product datasheet for the recommended diluent for your specific antibody.
Incubation Time
Primary antibody incubation according to a rigorously tested protocol provides consistent, reliable results. CST antibodies have been developed and validated for optimal results when incubated overnight at 4°C.
Detection System
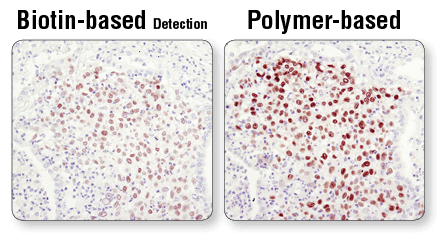
Polymer-based detection reagents, such as SignalStain® Boost IHC Detection Reagents (#8114 and #8125), in conjunction with SignalStain® DAB Substrate Kit (#8059), are more sensitive than avidin/biotin-based detection systems. Standard secondary antibodies directly conjugated with HRP may not provide sufficient signal amplification. In addition, always verify the expiration date of the detection reagent prior to use.
Polymer-based detection is more sensitive than biotin-based systems. IHC analysis of paraffin-embedded human lung carcinoma using Sox2 (D6D9) XP® Rabbit mAb #3579 and either biotin-based detection (left) or polymer-based detection (SignalStain® Boost IHC Detection Reagent #8114; right). As shown, polymer-based detection offers enhanced sensitivity and results in more robust staining.
Potential Problem: High Background
Slide Preparation
Inadequate deparaffinization may cause spotty, uneven background staining in IHC experiments. If this is the case, repeat the experiment with new tissue sections and using fresh xylene.
Peroxidase Quenching
Endogenous peroxidase activity in tissue samples may produce excess background signal if an HRP-based detection system is being used. In this situation, quench slides in a 3% H2O2 solution, diluted in RODI water, for 10 minutes prior to incubation with the primary antibody.
Biotin Block
Using biotin-based detection systems with samples that have high levels of endogenous biotin, such as kidney and liver tissues, can be problematic. In this case, use a polymer-based detection system such as SignalStain® Boost IHC Detection Reagents (#8114 and #8125). A biotin block may also be performed after the normal blocking procedure prior to incubation in primary antibody.
Blocking
To ensure adequate blocking in your IHC experiment, use 1X TBST (#9997) with 5% Normal Goat Serum (#5425) for 30 minutes prior to incubation with the primary antibody.
Antibody Dilution/Diluent
Product-specific recommendations for dilution and diluent are available on the data sheet and product page of all IHC-validated antibodies. Keep in mind that titration of the antibody may be required if a reagent other than the one recommended is used.
Secondary Cross-Reactivity
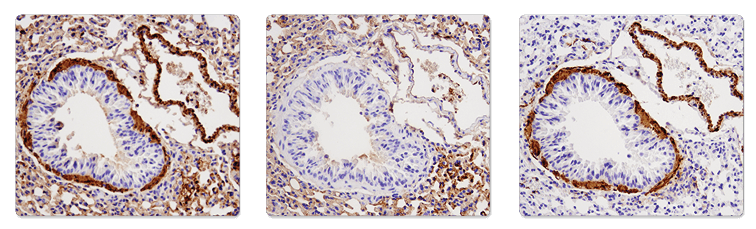
A secondary antibody may bind endogenous IgG, causing high background, in some samples where the secondary antibody is raised in the same species as the sample being tested (for example, in mouse-on-mouse staining). A control slide stained without the primary antibody should always be included to confirm whether the secondary antibody is the source of the background.
To find control slides for your target of interest, start here.
Immunohistochemical analysis of paraffin-embedded mouse lung using α-Smooth Muscle Actin (1A4) Mouse mAb (IHC Formulated) #56856 (left), SignalStain® Boost IHC Detection Reagent (HRP, Mouse) #8125 only (middle), or α-Smooth Muscle Actin (D4K9N) XP® Rabbit mAb #19245 (right). Note the presence of Mouse on Mouse background as a result of using anti-mouse secondary antibody on mouse tissue (left and middle). Use of anti-rabbit secondary reagent (SignalStain® Boost IHC Detection Reagent (HRP, Rabbit) #8114) eliminates the MOM background (right).
Washes
Adequate washing is critical for contrasting low background and high signal. Washing slides 3 times for 5 minutes with TBST (#9997) after primary and secondary antibody incubations is recommended.
Using Appropriate Positive and Negative Controls in Immunohistochemistry (IHC) Experiments
Control slides are a valuable tool that can be used to assess the performance of staining reagents and IHC methods. Each slide contains a positive and a negative pellet of formalin-fixed, paraffin-embedded (FFPE) cells.