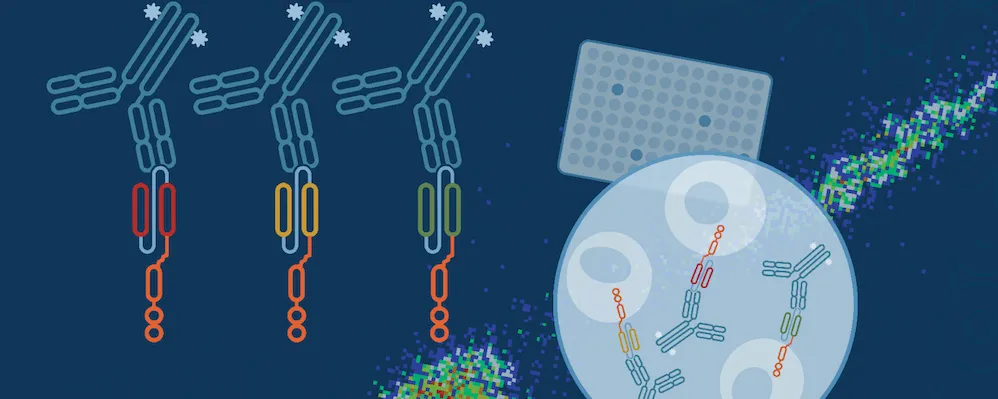
CAR-Engineered Cell Characterization Solutions
The development of chimeric antigen receptor (CAR) -based cell therapies is revolutionizing the treatment of human cancers and autoimmune diseases. As this innovative therapeutic modality continues to improve and become more accessible, better reagents are improving our ability to detect, identify, and fully characterize CAR-expressing cells. With a growing portfolio of innovative products, CST is helping to advance researchers' work in this field.
Characterize Your CAR-Engineered Cells Easier and Faster
One of the most significant challenges facing scientists developing and characterizing CAR-engineered cells is the need for robust, sensitive, and selective research reagents for the identification of CAR-engineered cells. If you plan to use an immunoassay platform in your research, developing your own anti-idiotype antibodies to identify CAR-engineered cells can be a long and resource-intensive process.
A Workflow Solution for Characterization of CAR-Engineered Cells
There are many stages in the complex process of characterizing CAR-engineered cells. CST focuses on one critical step in the process with a suite of purpose-built research tools for CAR cell characterization.
DETECT
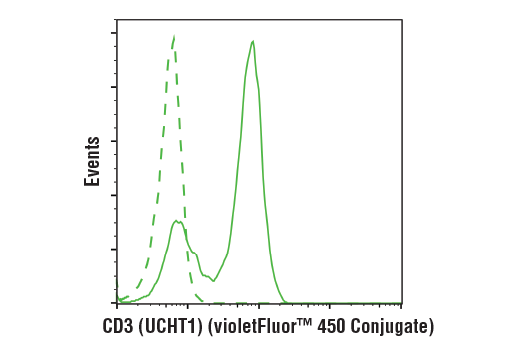
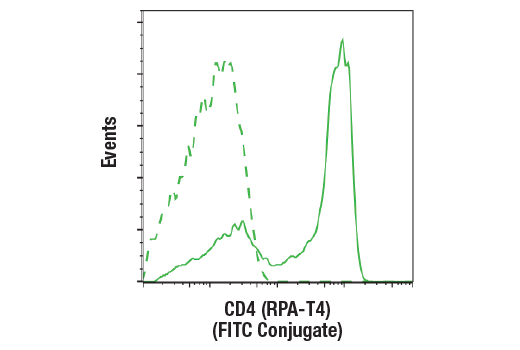
Evaluate CAR and target antigen expression
ANALYZE
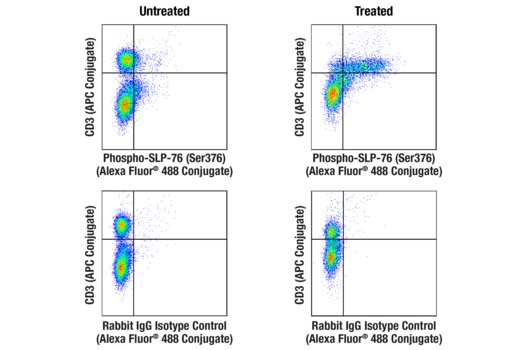
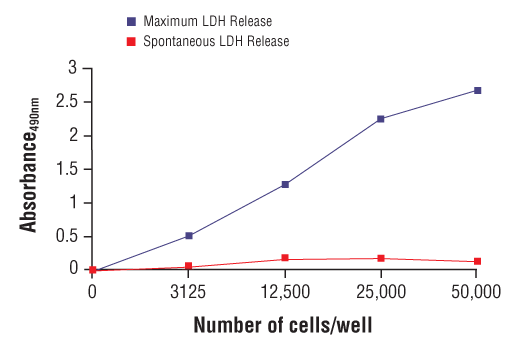
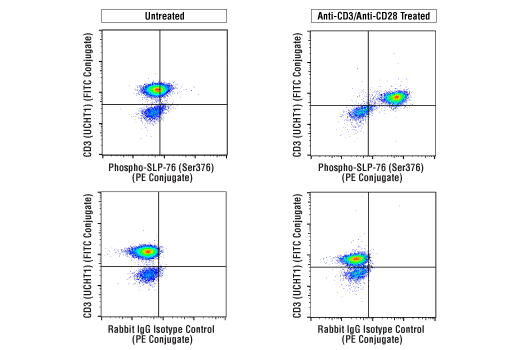
Interrogate immune cell activation, proliferation, viability, and signaling
QUANTITATE
Measure the density of CAR expression on the cell surface or the transduction efficiency of the CAR transgene
PURIFY
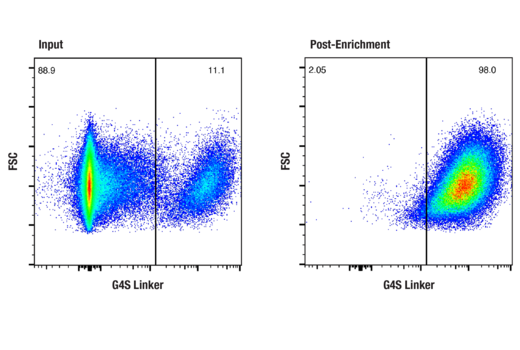
Enrich CAR+ cells using either bead-based or FACS-based sorting
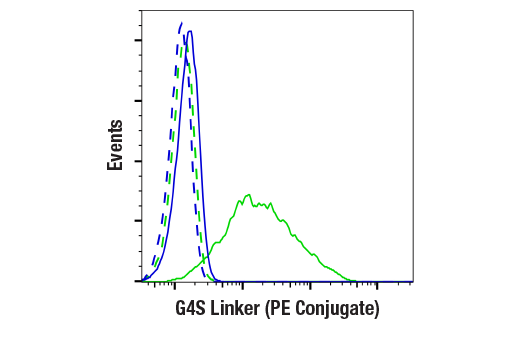
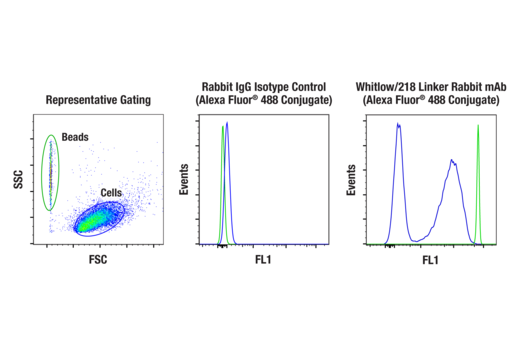
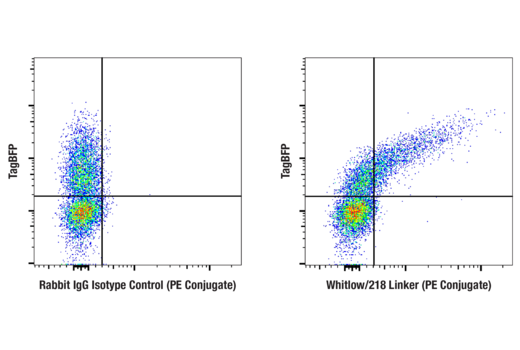
To streamline your characterization assays, CST scientists developed recombinant monoclonal antibodies to the ubiquitous peptide linker sequences of scFv-based CARs. By targeting these ubiquitous linker sequences, you can significantly reduce time, effort, and cost by eliminating the need for anti-idiotype antibodies to every CAR variant you’re testing. With high specificity to either the G4S or Whitlow/218 linkers, our CAR linker antibodies can detect the expression of scFv-based CARs, regardless of scFv specificity.
Video: Detecting CAR Expression with Versatile, Innovative Antibodies (2:04)
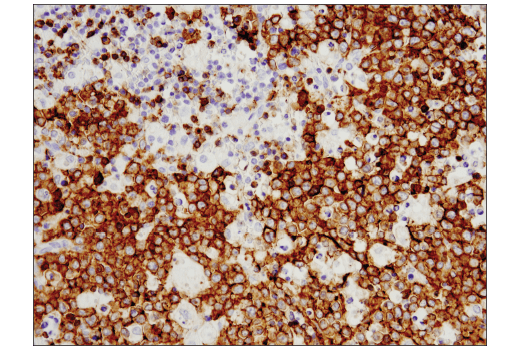
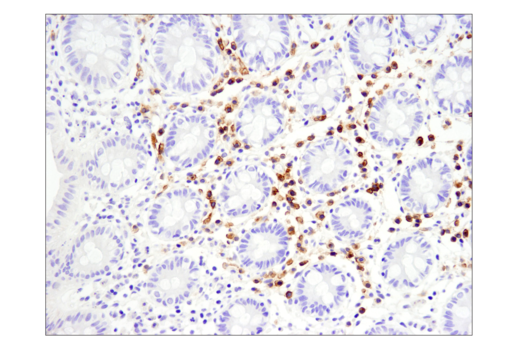
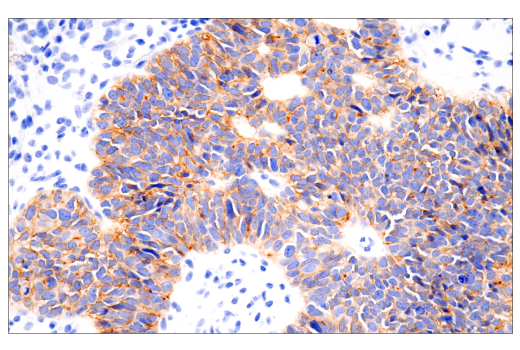
Histology Tools for Translational Research
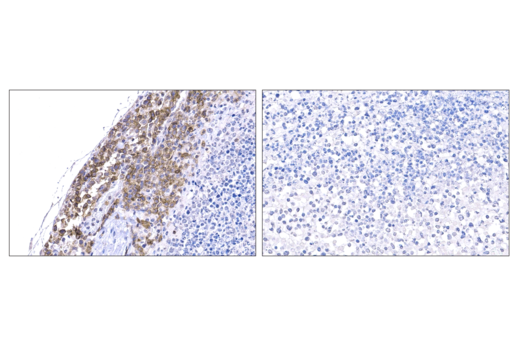
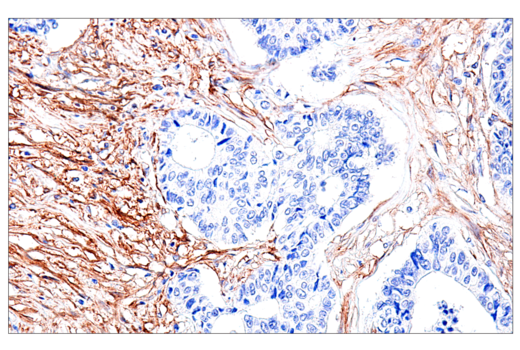
One of the most important questions scientists want to answer when challenging solid tumors with CAR-engineered cells is whether their therapeutic cells entered the tumor microenvironment. The most common methods include using either custom made anti-idiotype antibodies or RNA ISH, both of which have significant drawbacks. Direct interrogation of CAR expression in solid tumors using commercially available antibodies is now possible.
CAR-Engineered Cell Characterization Resources
Explore a growing library of Webinars, Scientific Posters, Application Notes, and other resources focused on CAR cell characterization.