Product Performance & Validation
Setting the world standard for antibody specificity, reproducibility, and performance since 1999.
For the last quarter-century, Cell Signaling Technology (CST) has set the standard for antibody performance by adhering to one immutable principle: do good science. As a company of dedicated scientists engaged in biological research ourselves, we know that you need research tools you can rely on to perform exactly as promised and give you consistent results you can trust. That’s why we’re so fanatical about product quality, and make every effort to ensure that anything we bring to market is the best it can be. Since our inception, no one has done more than CST to ensure reliable product performance. We’ll provide you with the right primary antibodies, secondary antibodies, ELISA kits, and immunoassay reagents for the experiments you’re doing—or we won’t sell them at all.
Every CST product is:
- Guaranteed to perform as expected every time
- Developed and made the right way, utilizing a sophisticated suite of the most advanced quality assurance technologies
- Never sold until we’re 100% sure it is specific and will perform correctly
This means that CST products deliver:
- Unsurpassed specificity—for unambiguous results
- Unparalleled consistency—with no variability
- Unmatched data quality—so you can have complete confidence in your results
- Faster time to results—so you can keep your projects moving forward
"It's no secret that we are considered at the very top of the industry in terms of the quality that we provide. The reason behind it is all the effort that we put into thoroughly validating the products that we sell. We’re also a research institution, so we're committed to real research, and we feel our customers’ pain because sometimes we also buy reagents that fail. Our culture is built upon doing good science when we validate tools that other people will buy and use. And that leads in the end to uncompromising business ethics. We simply refuse to sell bad stuff. We're not going to do that. We've been that way since we started and that's never going to change."
“I choose CST products based on published papers and colleague's recommendations. They're application-specific, and their results are specific and reproducible. I know I can work faster and better thanks to CST, because I don't have to worry about the antibodies' function and reproducibility.”
The CST Difference–In Their Words
How do we ensure optimum performance? Meticulous antibody validation is only the beginning.
Bench scientists—including those at CST—know that validation, testing, and quality control are not the same thing. You cannot substitute one for the other. It is critical that validation, testing, and QC each happen at the most appropriate points during production and development, and that they're conducted in the ways that are most relevant to the product and its intended use. At CST, we use many processes and techniques to ensure that our products are specific, consistent, and will perform as expected in your assay. Your experiments aren’t “one size fits all.” Neither is the way we prove the performance of our antibodies and other reagents.
Proven solutions—from our labs to yours.
At CST, we validate all of the antibodies we sell in-house using rigorous, application-specific testing. And we make 95% of the antibodies we sell. Having this kind of control over antigen design, antibody development, validation, and production is the best way we know to ensure that our products will consistently help you achieve reliable, reproducible results in your lab. And it’s why we can guarantee that CST® antibodies will perform as expected, every time.
Proof that the CST approach is the right one.
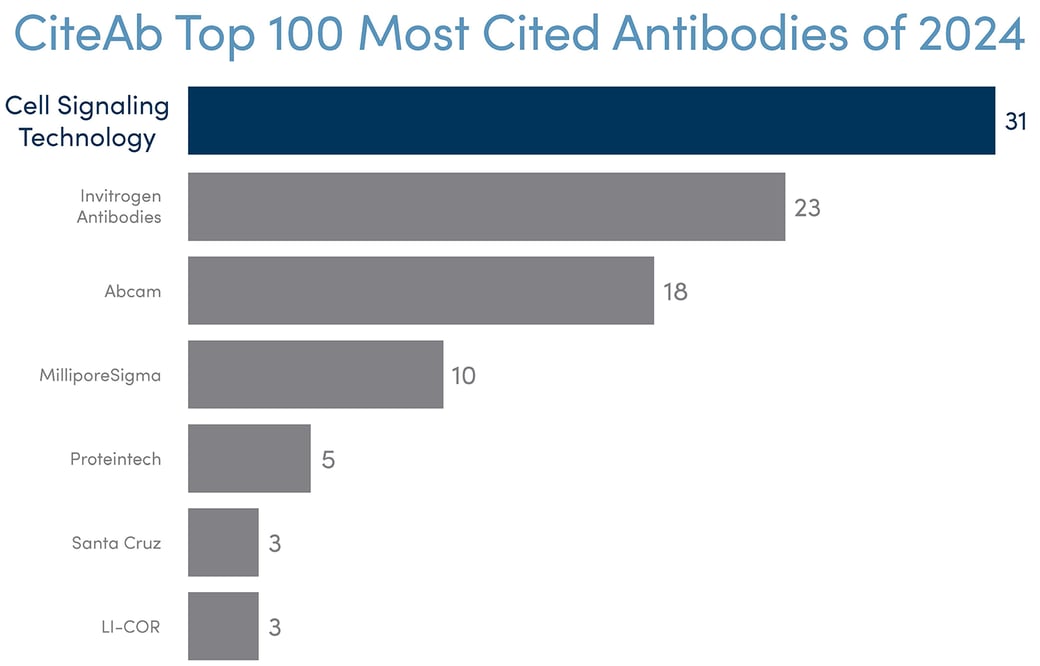
The results of our diligent, multi-faceted approach speak for themselves. During our 25 years, CST has earned more citations per antibody than any other company—by far. The three most cited antibodies of all time come from CST. And of the 100 antibodies most often cited in peer-reviewed publications, as detailed in CiteAb’s annual reports, nearly ⅓ are consistently from CST—more than any other supplier. That’s no accident. CST products are cited so frequently because researchers choose them knowing they'll get accurate, reproducible results to present or publish.
Our scientists have also published many more papers than those of any other antibody company. We do real science every day to understand the needs of our customers and ensure that you get the right products for the research you’re doing. And a large number of our scientists are application experts who’ve been developing products at CST for a decade or more. They know their reputation for excellence is on the line every day.
Antibody validation done right.
Your supplier’s antibody validation processes should be transparent to ensure their product performance meets your criteria. At CST, we view every antibody validation as a research project. Given the diversity of targets, samples, and applications, no two validation campaigns are exactly the same. Our scientists design a unique suite of experiments for every clone to confirm its performance—while considering the target’s biological context, the specifics of the application, and the sample types we think you’re likely to use. We will never recommend an antibody for an application in which it failed validation, so when you choose a CST application-recommended antibody, it’s going to work the first time and every time. Guaranteed.
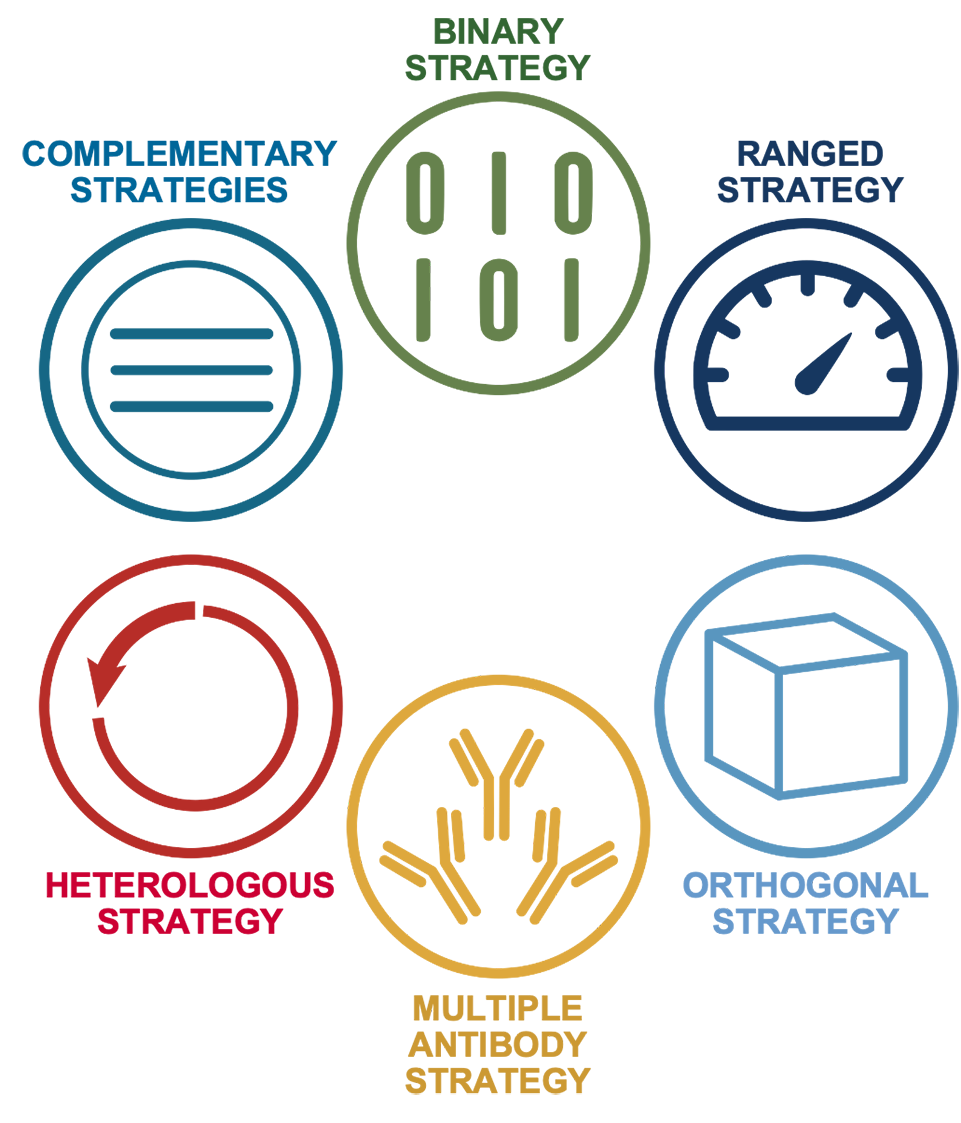
It all starts with the CST Hallmarks of Antibody Validation™ strategies.
CST employs a unique set of strategies for antibody validation in each given application. Inspired by the approaches outlined in “A Proposal for Validation of Antibodies” (Uhlen, et al., Nature Methods, 2016), the CST Hallmarks of Antibody Validation approach encompasses six complementary strategies that we use to design the optimal validation experiments for each antibody and application. These Hallmarks are often used in combination, resulting in a comprehensive data package that supports antibody performance.
No single assay is sufficient to validate an antibody, including knockout.
Our years of experience have shown that when assessing validation data, different application protocols can affect samples in unpredictable ways, especially in complex samples like tissue. That’s why we never assume that a positive result—or lack of one if the target is knocked out—in a given application means the antibody will be specific in every application. From flow cytometry to immunohistochemistry, immunofluorescence, western blotting, immunoprecipitation and others, no antibody supplier in the world has higher standards for antibody specificity, reproducibility, and performance.
Depending on the application, sample, biological context, and other factors, we draw from the most appropriate experimental models to confirm antibody specificity, sensitivity, and suitability.
| Relevant Applications | Experimental Models | Hallmark Strategy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| WB/IP | IHC | IF | FLOW | ChIP | ELISA | ||||
| ◉ | ◉ | ◉ | ◉ | ◉ | ◉ | Positive & negative cell lines or tissues | BINARY | ||
| ◉ | ◉ | ◉ | ◉ | ◉ | ◉ | Knockout/CRISPR | |||
| ◉ | Positive & negative loci | ||||||||
| ◉ | ◉ | ◉ | ◉ | ◉ | High & low expressing cell lines or tissues | RANGED | |||
| ◉ | ◉ | ◉ | ◉ | ◉ | ◉ | Treatments to induce or inhibit protein expression or PTM | |||
| ◉ | ◉ | ◉ | Treatments to modulate protein localization | ||||||
| ◉ | ◉ | ◉ | ◉ | ◉ | Knock-down, siRNA/shRNA | ||||
| ◉ | ◉ | Tissue arrays | |||||||
| ◉ | ◉ | ◉ | ◉ | ◉ | Comparison to non-antibody derived data (ISH, Mass Spec) | ORTHOGONAL | |||
| ◉ | ◉ | ◉ | ◉ | ◉ | ◉ | Comparison to omics profiles | |||
| ◉ | ◉ | ◉ | ◉ | ◉ | ◉ | Comparison of results obtained with multiple antibodies with non-overlapping epitopes | MULTIPLE ANTIBODY | ||
| ◉ | IP-western performed with unique antibodies | ||||||||
| ◉ | ◉ | ◉ | ◉ | ◉ | Protein overexpression | HETEROLOGOUS | |||
| ◉ | ◉ | Purified recombinant protein | |||||||
| ◉ | ◉ | ◉ | ◉ | ◉ | ◉ | Use of site-specific mutants | |||
| ◉ | Comparison of results from multiple targets within the same protein complex | COMPLEMENTARY | |||||||
| ◉ | ◉ | Peptide arrays & ELISA to verify PTM specificity | |||||||
| ◉ | ◉ | ◉ | ◉ | Peptide competition assays | |||||
| ◉ | ◉ | ◉ | ◉ | Verification of modification specificity in cell lines or tissues | |||||
Insist on application-specific validation.
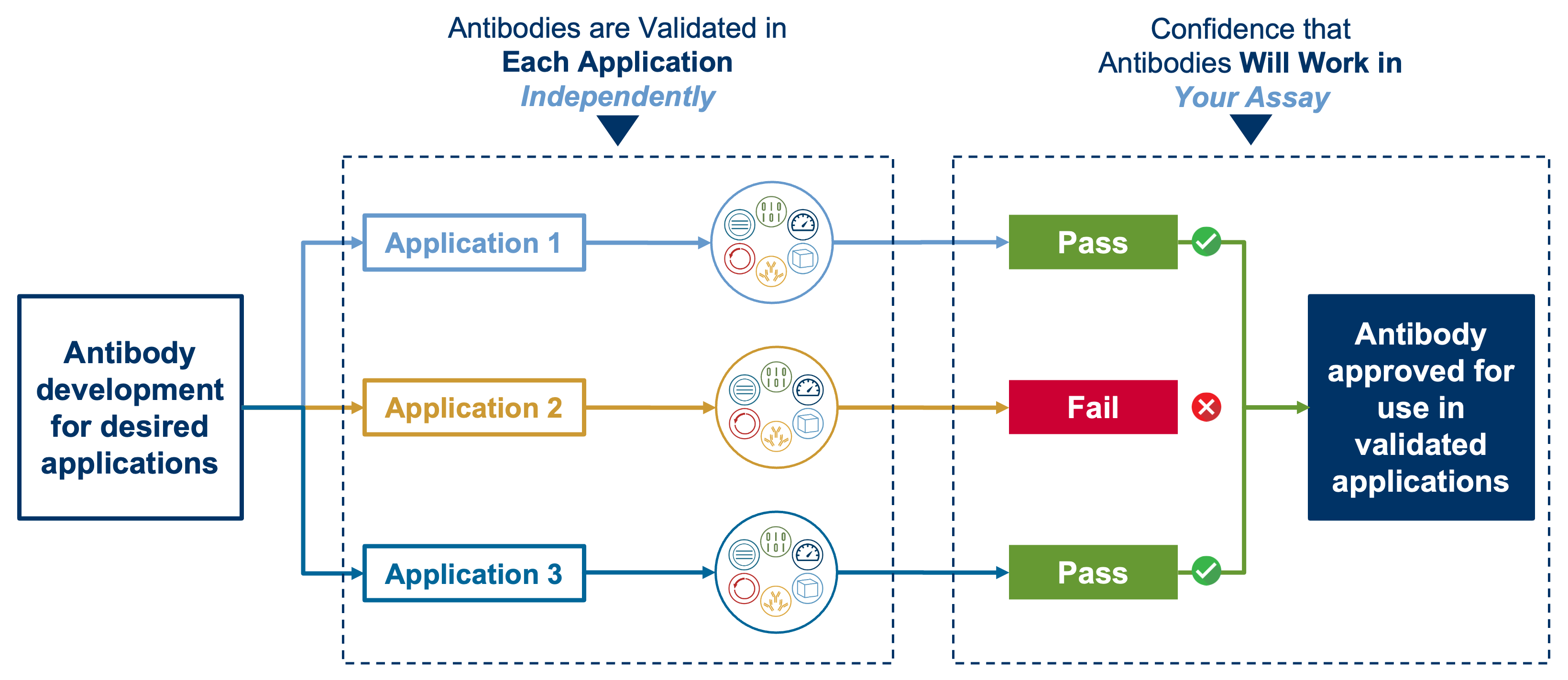
The CST validation process includes everything required to take a new antibody clone from development to commercialization. Because we understand that antibodies can perform differently under different experimental conditions, we validate our products in each application independently, employing assays from one or more of the Hallmarks of Antibody Validation approach. This painstaking approach helps ensure that you won’t experience non-specific binding and other performance issues when using CST antibodies.
Our promise to you:
When we recommend an antibody for an application, you can be confident that it will work in your assay. Our goal is to prevent false positives—and false negatives—in your results. The following examples illustrate how critical it is to validate antibody specificity in each anticipated application (or sample type) separately.
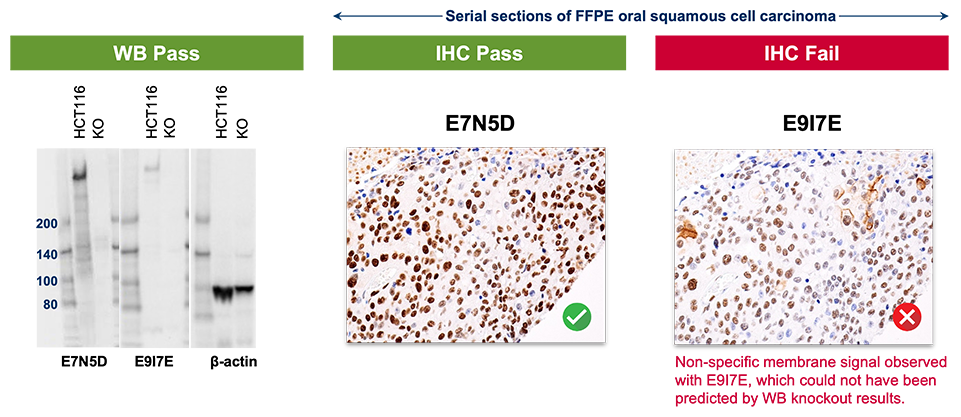
1. Knockout specificity in WB does not predict specificity in IHC.
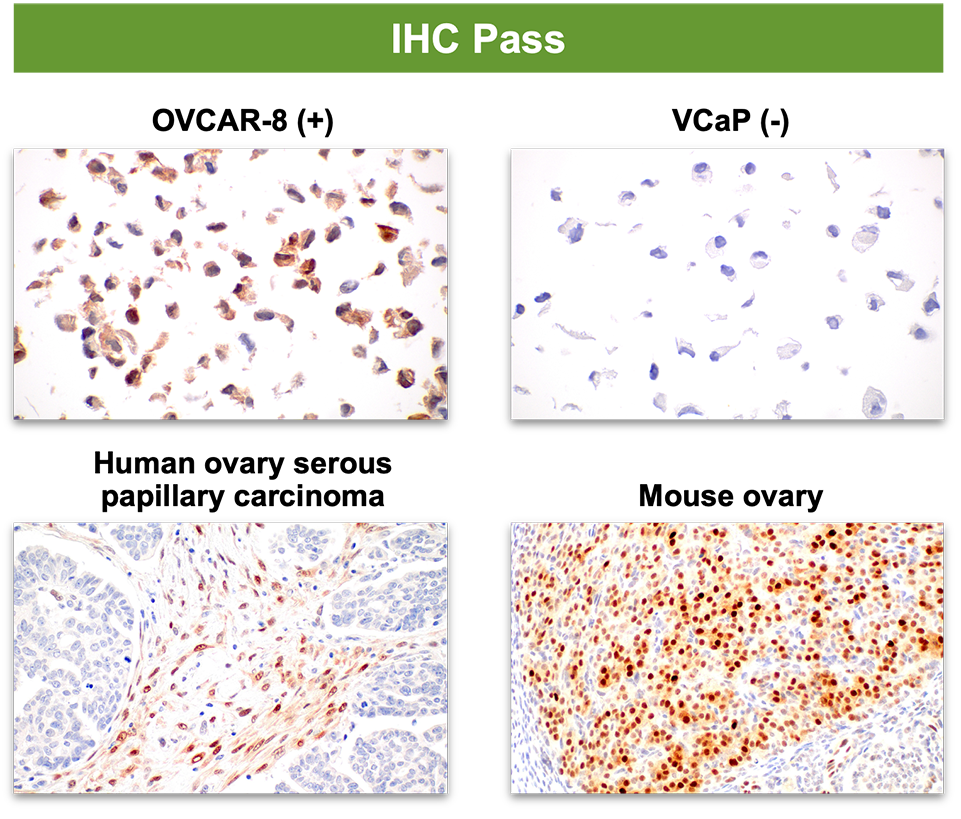
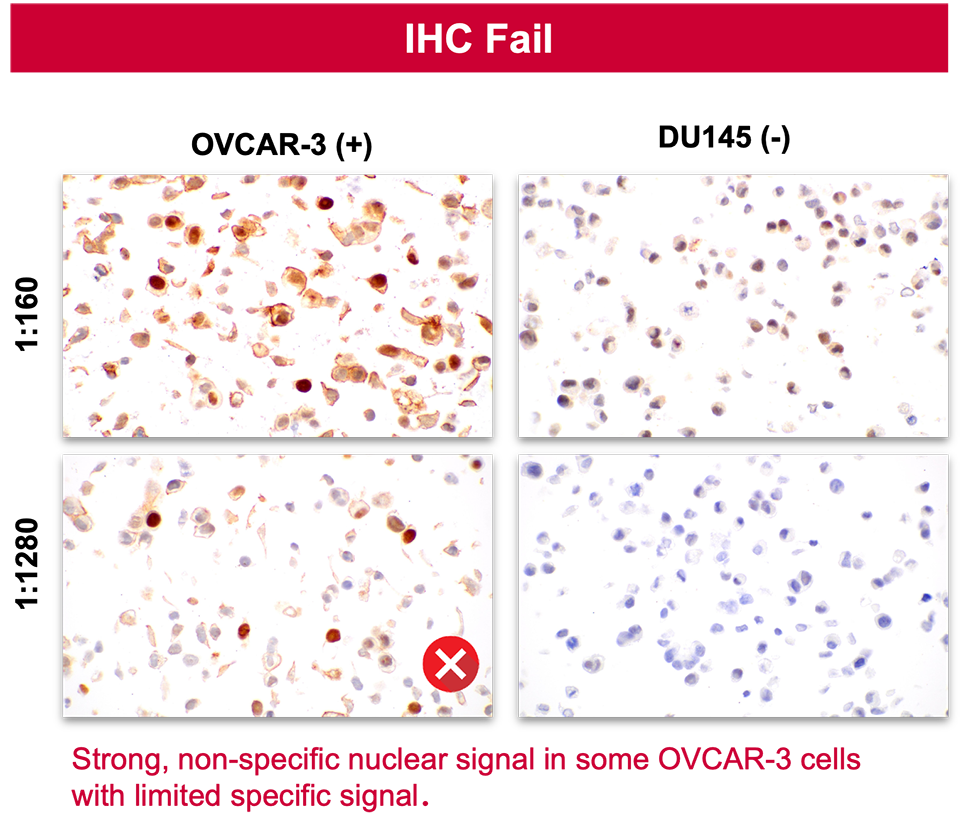
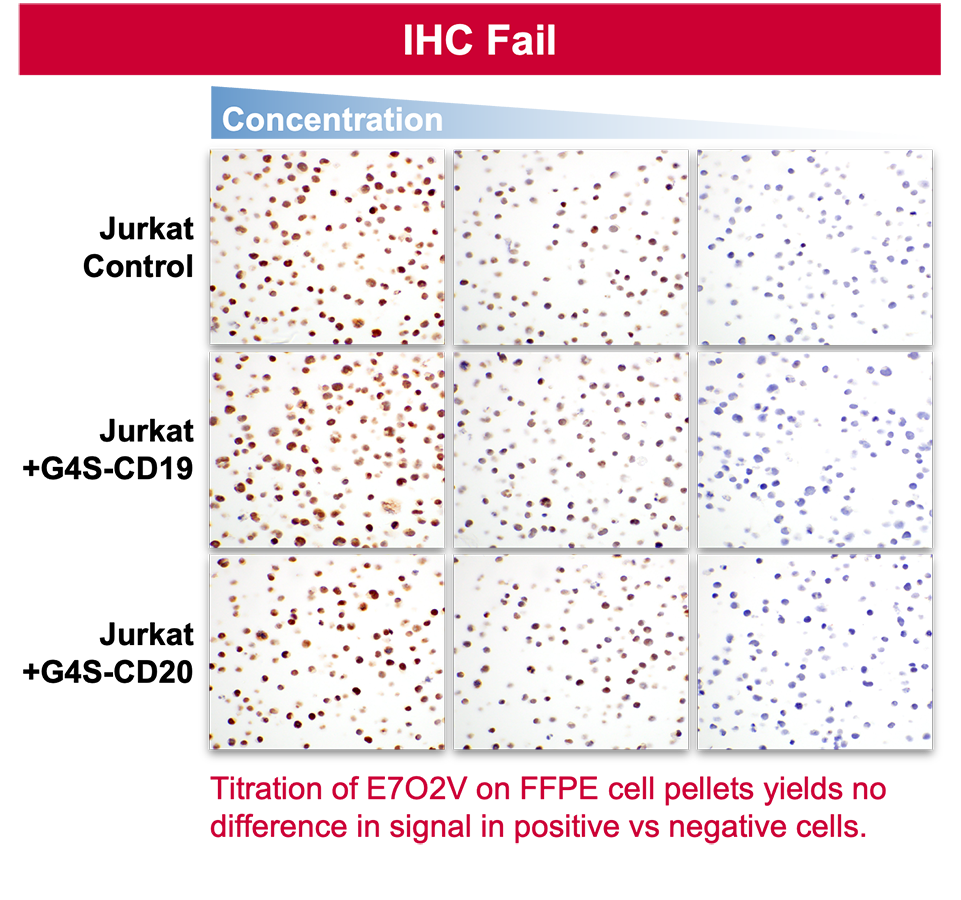
2. Specificity in IHC does not predict specificity in IF.
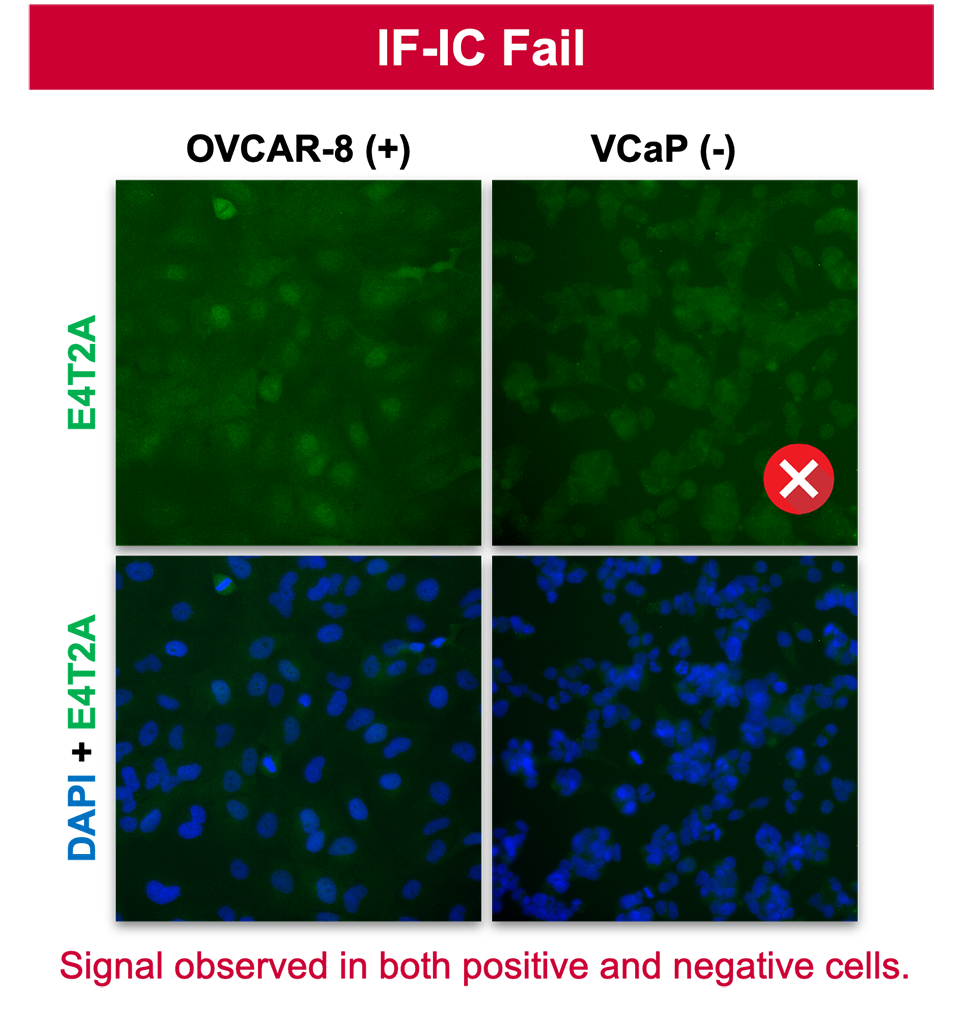
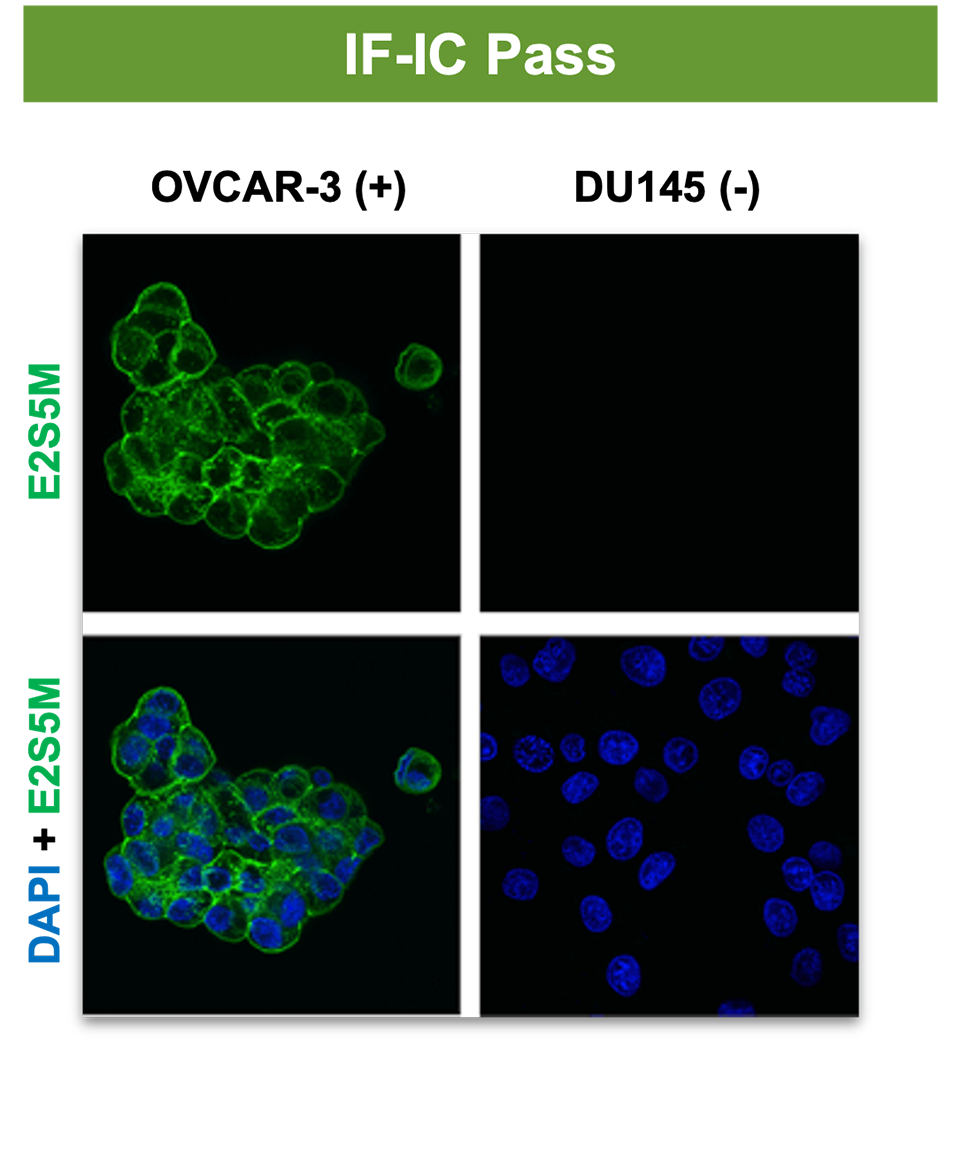
3. Specificity in IF-IC does not predict specificity in IHC.
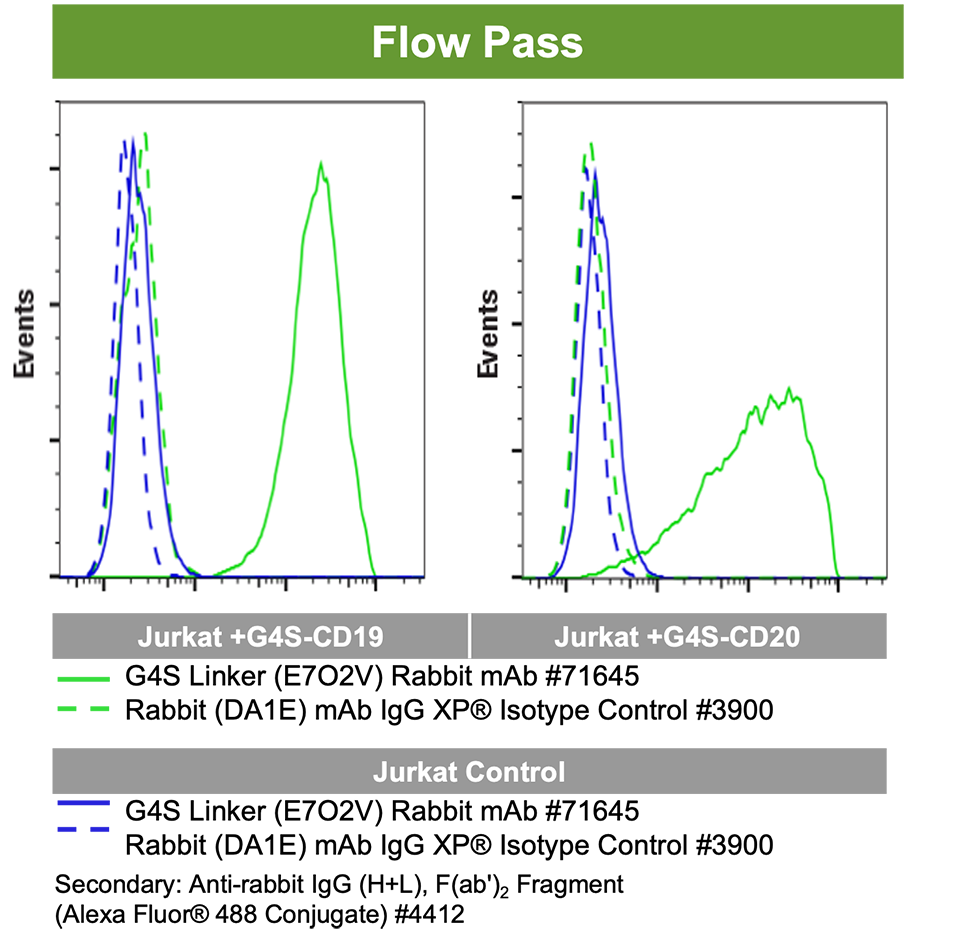
4. Specificity in Flow does not predict specificity in IHC.
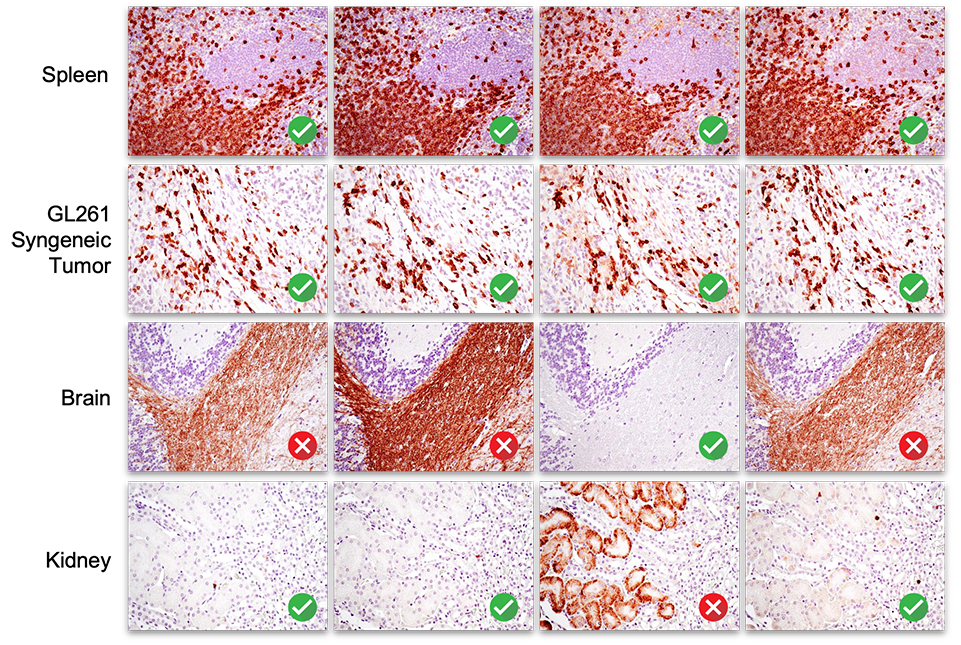
5. Signal in some tissues does not predict specificity overall.
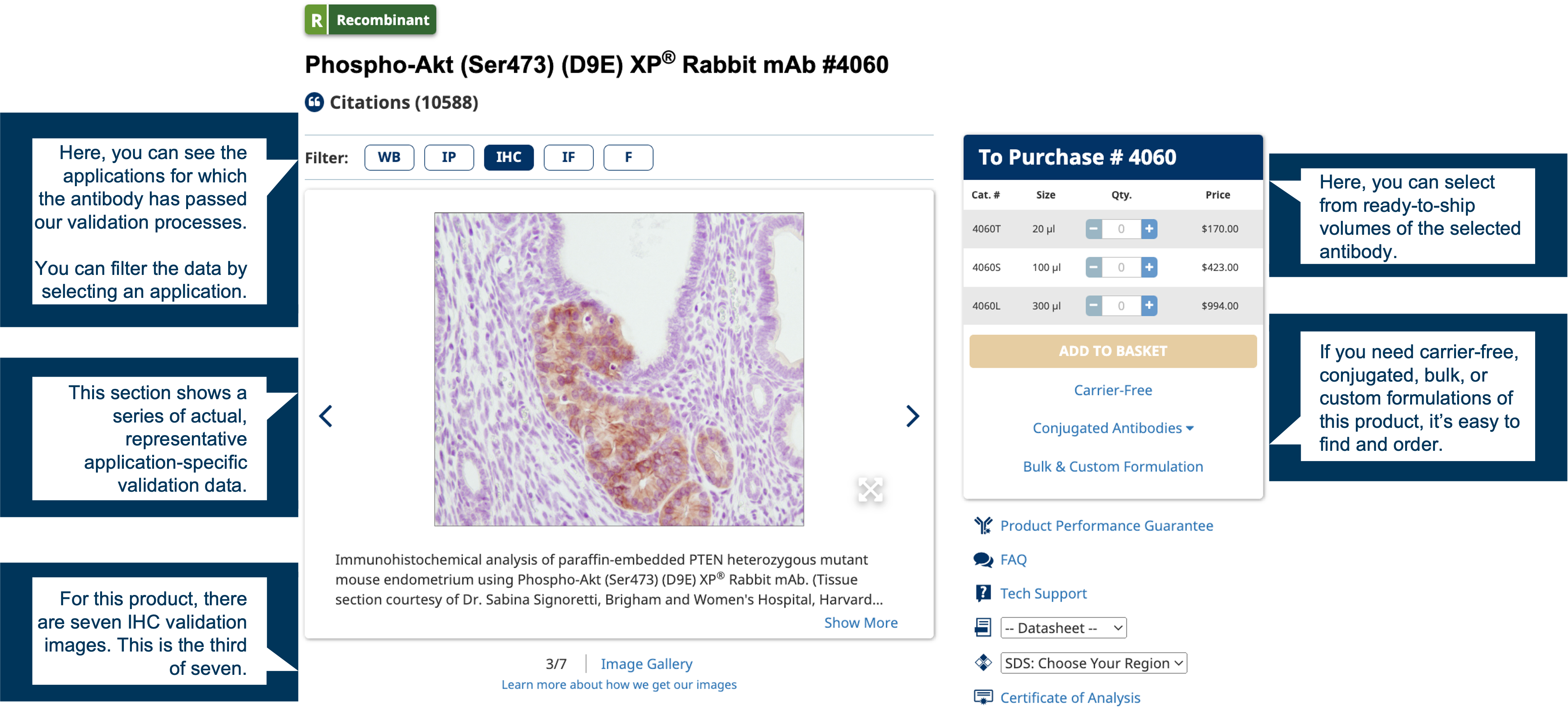
Where’s the validation data? We show it for every product.
When we offer an antibody for sale, it has already been through the most stringent validation process in the industry. Take a look at any product on our website: you’ll see a variety of real application data examples. If the application you’re interested in isn’t represented, the antibody either didn’t meet our performance standards for that application, or we simply didn’t test it at the time. If you’d like to know which is the case, just ask us. We will provide you with any data we have that can help you assess the suitability of the product for your project. Similarly, if your species or model of interest isn’t represented, we may be able to help you determine what is likely to work and what isn’t. Visit our Technical Support page to find out more.
The data images and graphs we show for each product are representative of its expected performance, not just “the best of the best” results we generated. We will never present data that could lead you to draw the wrong conclusion in your research. The performance you see for each of our products is what you can expect when you use them in your own lab.
For more than 25 years, CST products have measured up to the highest standards. Ours.
Our commitment to doing good science and our painstaking approach to ensuring product quality means that you can trust CST to provide you with products you know will perform as expected every time. We’d rather have our antibodies fail at our bench than yours.
You would never rely on a single data point to make your conclusions, and neither do we. We do everything we can to break the antibody’s performance. You may be surprised to learn that more antibodies fail in our development and validation process than pass. This ensures that only the most specific, best-performing antibodies make it to your lab.