Identifying Myeloid Subsets Using Antibodies
Development of Myeloid Cells
During hematopoiesis the Common Myeloid Progenitor (CMP) cell is the earliest progenitor cell that specifies a myeloid lineage for its progeny. The CMP cell can be characterized by its unique immunophenotype. The CMP gives rise to following terminally differentiated myeloid cell types:
Monocytes are produced in the bone marrow and circulate in the bloodstream for up to three days before migrating into tissues where they differentiate into macrophages and dendritic cells. Monocytes are a part of the innate immune system but also influence adaptive immunity through antigen presentation and cytokine production upon differentiation into macrophages and dendritic cells.
Macrophages recognize antigens such as damaged cells or foreign material and are present in most tissues for immediate destruction of antigens via phagocytosis, a process that can also lead to antigen presentation. Upon tissue injury or pathogen infection, monocytes in the blood are recruited to the affected tissue and differentiate into macrophages.
Dendritic cells have the capacity to engulf cellular and foreign material by phagocytosis and process the material for presentation as antigens to T Cells. As such, dendritic cells serve to relay information about pathogens between the innate and adaptive immune systems.
Neutrophils are referred to as granulocytes due to the characteristic granules in their cytoplasm. In neutrophils, the granules act to release toxic compounds in response to the presence of pathogens such as fungi and bacteria. Like dendritic cells, they can also phagocytose and process antigens for antigen presentation.
Eosinophils are produced in the bone marrow from granulocyte-macrophage progenitor (GMP) cells and traffic via the circulatory system mainly to the gastrointestinal tract but can also be attracted to inflammatory sites. Eosinophils function in the innate immune response to parasites and viral infections, and are also implicated in controlling allergic responses.
Mast cells are produced from GMP cells and traffic via the circulatory system to most tissues, where they complete their maturation. Mast cells function in wound healing and provide protection against pathogens by secreting cytokines as well as granules containing histamine and heparin. This mediates further immune responses such as macrophage recruitment and blood vessel dilation.
Basophils are granulocytes that complete maturation in the bone marrow before entering the circulatory system and only migrate into peripheral tissues in pathological settings. Basophils have a well-established role in protective immunity to helminth parasites, enhancing B Cell responses to respiratory bacteria and have also been implicated in the initiation of T-helper cell (Th2) adaptive immune responses
Megakaryocytes are derived from CMP cells and are polyploid and granulated due to their ability to replicate DNA and the inability to divide. These mature megakaryocytes extend long branching processes called pro-platelets that emit platelets into circulation via the sinusoidal blood vessels of the bone marrow.
Erythrocytes are derived from CMP cells and undergo several rapid cell divisions during development. This results in an enormous population expansion coupled with a progressive reduction in cell size, as the cell sheds most organelles to produce a mature erythrocyte. Erythrocytes, in addition to supplying oxygen for tissues, can regulate vascular tone and provide an immune function by producing cytotoxic reactive oxygen species from hemoglobin released from cells lysed by pathogens.
Platelets are small anucleated cells derived from megakaryocytes. Platelets are effectors of hemostasis that stop bleeding by forming a platelet plug. Platelets are also recruited to sites of infection and potentially modulate inflammatory processes by interacting with leukocytes and by secretion of inflammatory mediators.
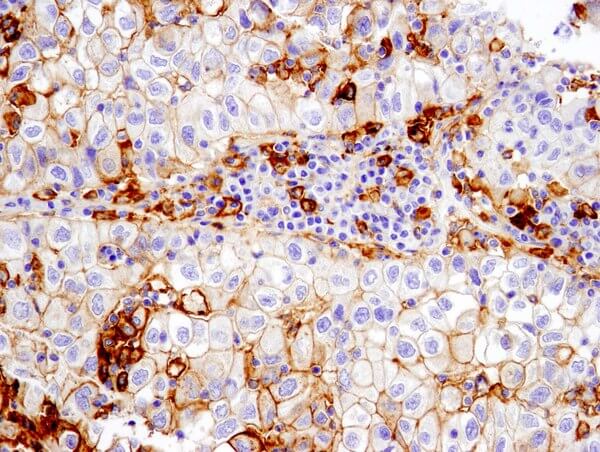
PD-L1 (E1L3N®) XP® Rabbit mAb #13684: IHC analysis of paraffin-embedded human non-small cell lung carcinoma using #13684 performed on the Leica Bond Rx.
Myeloid Cell Immunophenotyping
Groups of antibodies with similar binding patterns to leukocyte cell surface molecules at various stages of differentiation are used to identify “cluster of differentiation” (CD) antigens. These patterns identify individual classes of immune cells and can be used to detect the presence of a specific immune cell in a heterogeneous population.
Correctly identifying the immunophenotype can have significant consequences, as in heterogeneous tumors where distinct tumor populations may exhibit varying sensitivities to different cancer treatments.
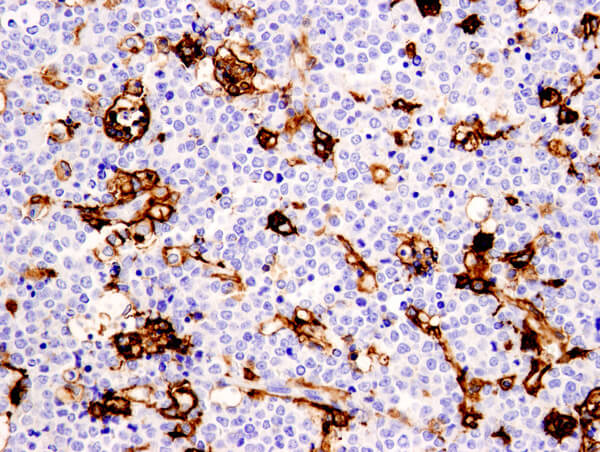
CD11c (D3V1E) XP® Rabbit mAb #45581: IHC analysis of paraffin-embedded human non-Hodgkin's lymphoma using #45581.
Myeloid Marker Selection for Immunohistochemistry (IHC)
Immunohistochemical immunophenotyping allows evaluation of the expression and tissue architecture of a protein. IHC observations can provide additional information regarding patterns of involvement and the cytological features of cells.
Recent studies suggest that macrophages do not form stable subsets but respond to a combination of factors that interact to form a complex, possibly mixed, phenotype. Alternate states of macrophage activation, referred to as M1 & M2, have been identified. Pathological conditions can lead to the development of mixed M1/M2 phenotypes whose composition is dependent on a balance of activating and inhibitory activities, as well as the tissue environment.
This points to the need for bullet-proof antibody validation for immunophenotyping. CST has a wide array of expertly validated antibodies that can distinguish immune cell populations accurately and reproducibly.
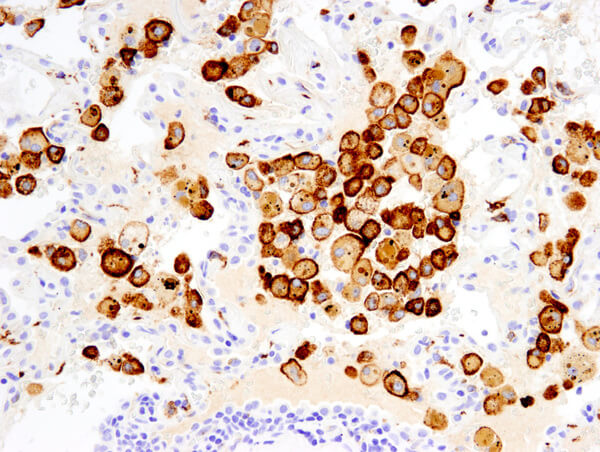
CD206/MRC1 (E2L9N) Rabbit mAb #91992: IHC analysis of paraffin-embedded human non-small cell lung carcinoma using #91992 performed on the Leica Bond Rx.