Cellular senescence is characterized by irreversible cell-cycle arrest in combination with a distinct secretory phenotype and expanded lysosomes in response to stress. Senescent cells accumulate in tissues during aging, and various markers of senescence are associated with neurodegenerative disease. p16 accumulates in senescent astrocytes and microglia in the context of Tau pathology. p16 and p27 accumulate in senescent oligodendrocyte progenitor cells (OPCs) in the context of amyloid plaque pathology. Senolytic compounds are investigated to remove senescent cells and treat Alzheimer’s disease.
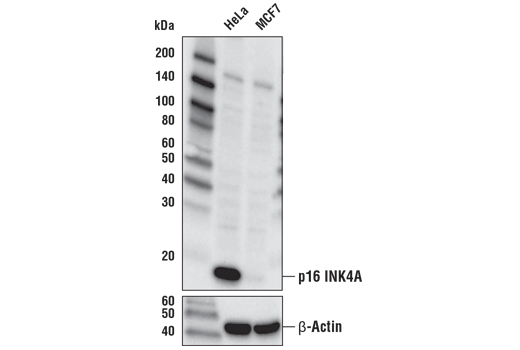
p16 is a member of the INK4 family of cyclin-dependent kinase inhibitors that are responsible for arresting the cell cycle in the G1 phase. p16 is commonly used as a marker for senescent cells. Elevated expression of p16 has been observed in the neurons of Alzheimer’s disease patients and may also play a role in the progression of multiple sclerosis.
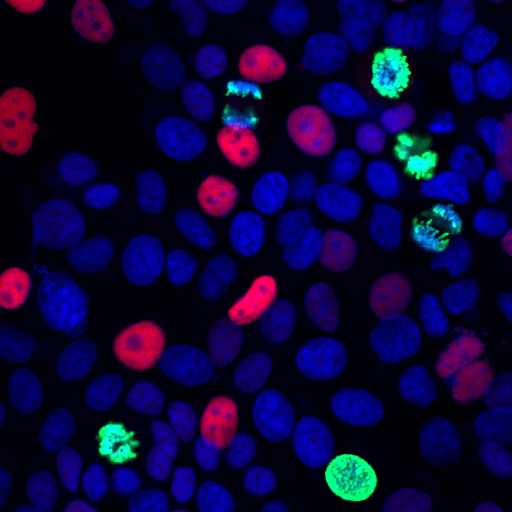
p21 Waf1/Cip1, a CDK inhibitor, is a common marker of cellular senescence. It causes cell cycle arrest in response to stress-induced p53 to trigger senescence. p21 may be a critical mediator of cell cycle dysregulation in Alzheimer’s disease.
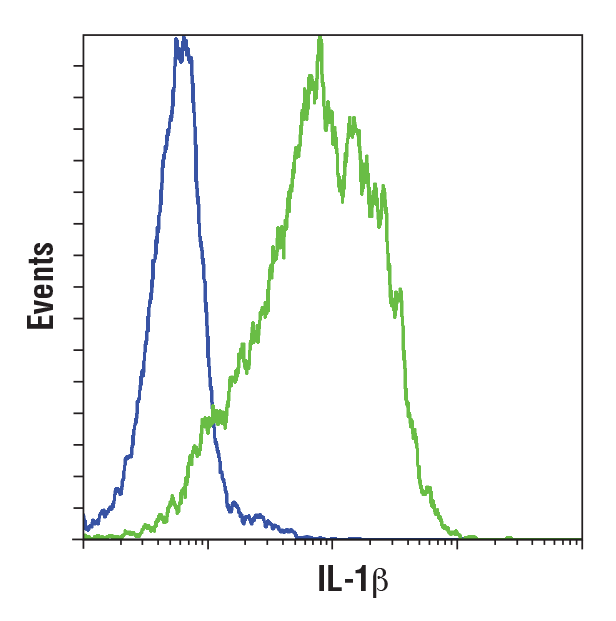
IL-1β is a pro-inflammatory cytokine that is secreted by senescent cells. It is elevated in Alzheimer’s disease brain tissue, cerebral spinal fluid (CSF), and serum, potentially due to increased p38MAPK activity. Aged in vitro rat microglia may acquire a senescent phenotype characterized by increased levels of IL-1β and TNF-α after treatment with beta-amyloid oligomers. Elevated levels of IL-1β have also been observed in the CSF, serum, and dopaminergic regions of the striatum from patients with Parkinson’s disease.
TNF-α is a cytokine whose dysregulation has been implicated in Alzheimer’s disease and Parkinson’s disease. Levels of TNF-α are increased in the brain tissue, CSF, and serum of Alzheimer’s disease patients, potentially due to increased p38MAPK activity. Aged in vitro rat microglia may acquire a senescent phenotype characterized by increased levels of IL-1β and TNF-α after treatment with beta-amyloid oligomers. Elevated levels of TNF-α have also been observed in the CSF, serum, and dopaminergic regions of the striatum from patients with Parkinson’s disease.
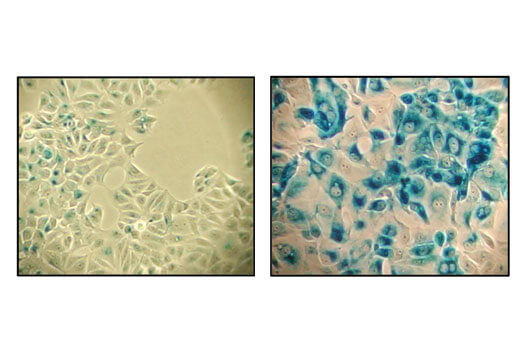
Senescent cells can be identified by an increased level of lysosomal β-galactosidase activity. Beta-amyloid triggers senescence in in vitro models, driving expression of p16 INK4A and senescence-associated β-galactosidase. Increased β-galactosidase activity has also been observed in the cerebrospinal fluid of Parkinson’s disease patients.