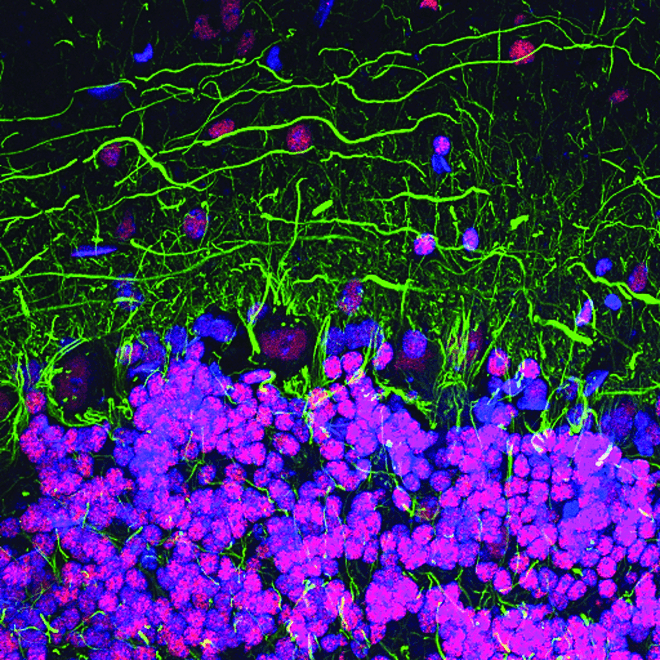
Abnormal cell-cell communication, for example disrupted presynaptic input, as well as disrupted intracellular signaling contribute to the pathogenesis of neurodegenerative disease. Understanding the signal transduction pathways that regulate gene expression will help efforts to develop therapeutic interventions.
CREB signaling is a cellular transcription factor that plays an important role in the formation of memories. Perturbed signaling has been observed in the brains of Alzheimer’s disease mouse models, suggesting CREB signaling may be disrupted in human Alzheimer’s disease brains as well. Disturbances in CREB function may also contribute to the development and progression of Huntington’s disease.
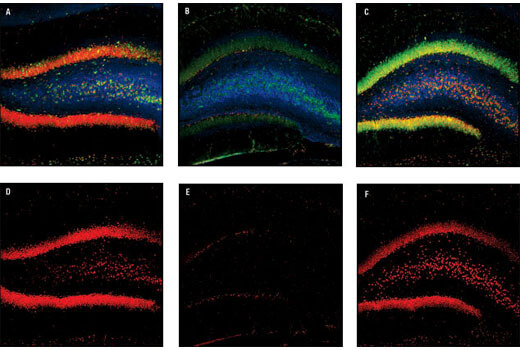
CREB is a cellular transcription factor activated when phosphorylated on Ser133. pCREB (Ser133) levels are reduced in the prefrontal cortex of patients with Alzheimer’s disease, indicating a dysfunction in CREB signaling. Reduced pCREB levels in the peripheral blood mononuclear cells (PBMCs) of patients with Alzheimer’s disease correlate with pCREB levels observed in postmortem Alzheimer’s disease brains, suggesting pCREB expression in PBMCs may be a potential biomarker for disease progression.
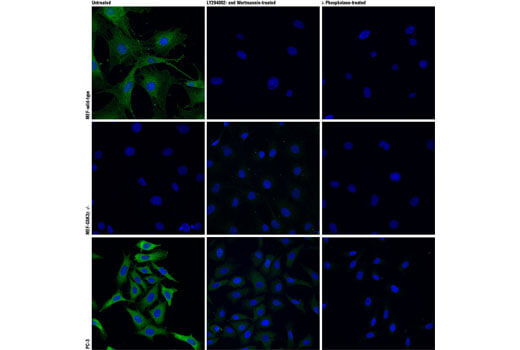
GSK-3β is known to interact with Tau, beta-amyloid (Aβ), and α-synuclein and is implicated in the pathogenesis of Alzheimer’s disease and Parkinson’s disease. It is one of the kinases responsible for Tau hyperphosphorylation, resulting in neurofibrillary tangles. GSK-3β regulates several critical cellular events, such as axonal transport, microtubule dynamics, apoptosis, and inflammation, making GSK-3β a potential therapeutic target.
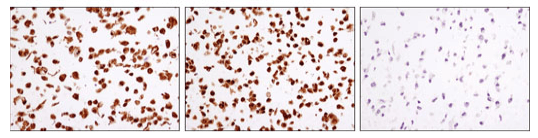
Phosphorylation of GSK-3β on Ser9 inactivates the protein, influencing its ability to regulate glycogen synthesis in response to insulin. In Alzheimer’s disease mouse models, GSK-3β Ser9 phosphorylation may also reduce APP processing by β-secretase, decreasing Aβ production.