One hallmark of many neurodegenerative diseases is the accumulation of unfolded or misfolded proteins that lead to neurofibrillary tangles and plaques that cause neuronal cell cytotoxicity. There is increasing interest in the field on understanding the mechanisms required for the production and processing of proteins known to form these aggregates, as these protein aggregates are associated with Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis. Some challenges to developing new therapies that target the protein aggregate formation include an incomplete knowledge of the mechanisms of action as well as a lack of biomarkers to diagnose conditions early and to monitor disease progression and therapeutic response.
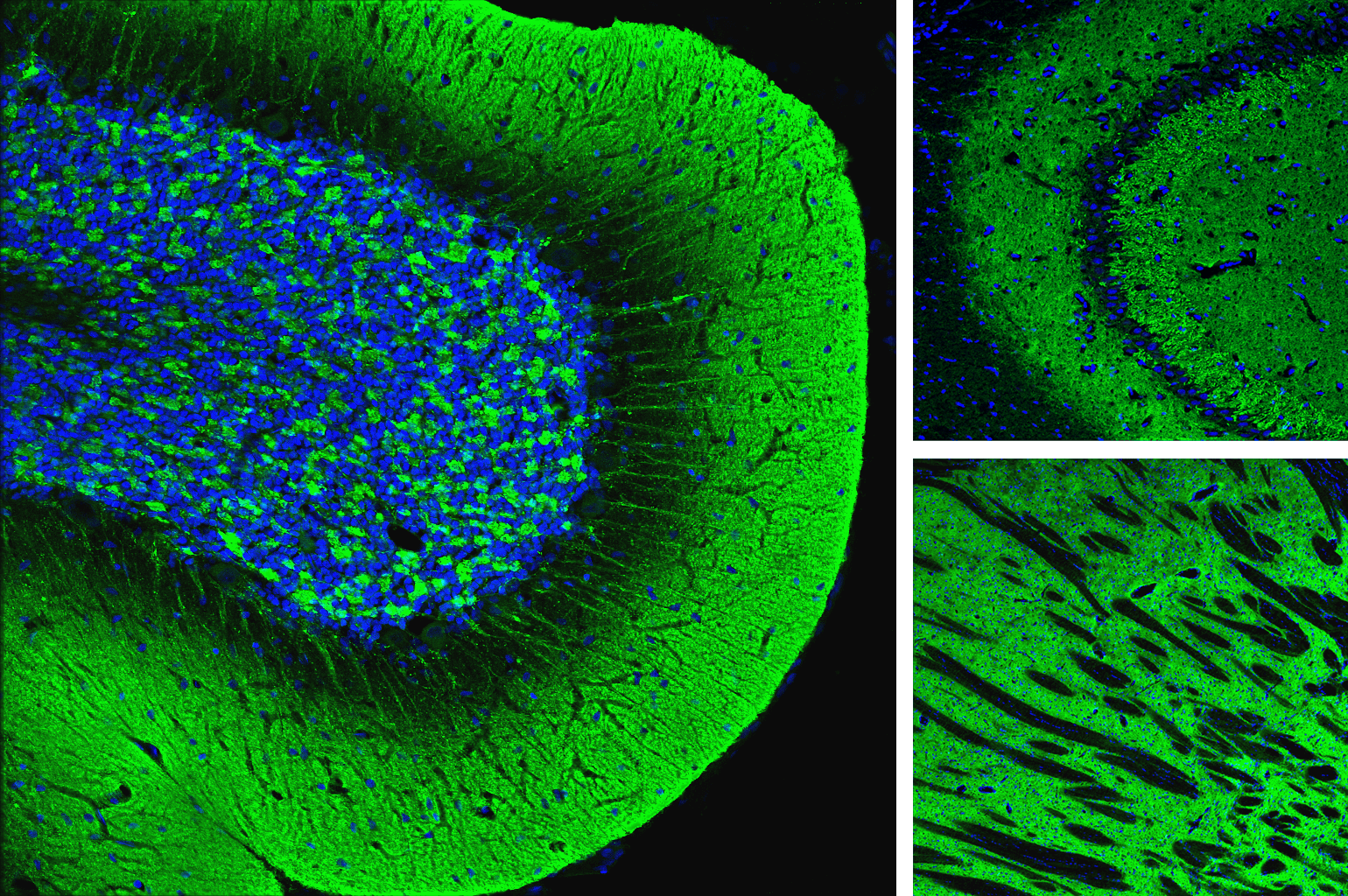
Beta-amyloid (Aβ) is a peptide that is the main component of the amyloid plaques observed in the brains of patients with Alzheimer’s disease. The peptides are formed when the amyloid precursor protein (APP) is cleaved by β-secretase and γ-secretase.
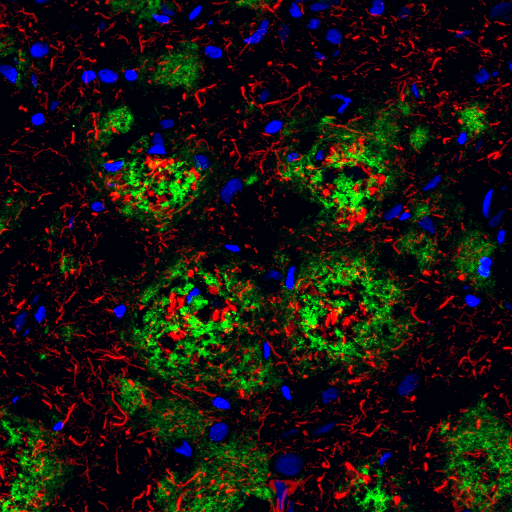
Under normal cellular conditions, Tau promotes and stabilizes microtubule assembly, especially in axons. Phosphorylation of Tau (Thr205) is well characterized in neurofibrillary tangles, with low levels in earlier stages and significantly higher levels in later stages of Alzheimer’s disease.
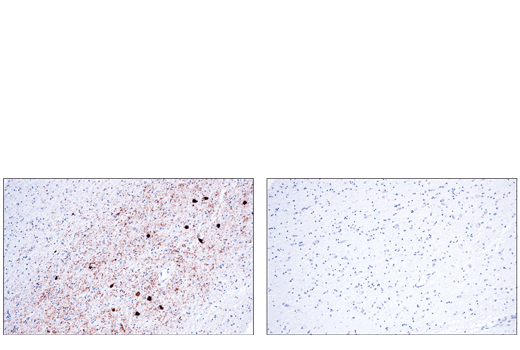
Interest in α/β-synuclein began when mutations were observed in several families with autosomal-dominant Parkinson’s disease. α/β-synuclein may regulate membrane stability and/or turnover; however, its normal cellular function has not been conclusively determined. α/β-synuclein mutations are associated with early onset familial Parkinson’s disease.