Acquiring Cell Death
Mutations in cell death pathways, such as apoptosis, mitophagy, necroptosis, and autophagy, contribute to neuronal cell death and the progression of neurodegenerative diseases. Aberrant pro- and anti-apoptotic signaling, mitochondrial dysfunction, mis-regulation of autophagy or the unfolded protein response, and activation of the necrosome by stress and/or inflammation highlight just a few of the mechanisms by which neurons die or become diseased. Although many of these pathways are understood in non-neuronal cells, their mechanism of activation and dysregulation remains a mystery in neurons which present their own challenges.
Start with these targets
Cleaved PARP (ASP214)
PARP typically functions as a key player in the DNA repair pathway in response to oxidative stress. When cleaved by caspase 3 between Asp214 and Gly215, the N-terminal cleaved fragment inhibits DNA repair enzymes to push neurons toward apoptosis, making it a hallmark of apoptotic cells.
Products
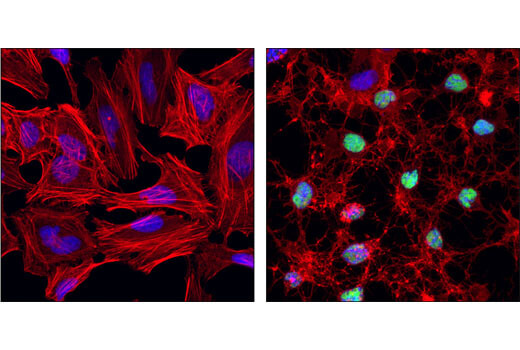
Cleaved PARP (ASP214) (D64E10) XP® Rabbit mAb #5625 – IF
Cleaved PARP (ASP214) (D64E10) XP® Rabbit mAb #5625: Confocal immunofluorescent analysis of HeLa cells, untreated (left) or treated with Staurosporine #9953 (right), using Cleaved PARP (Asp214) (D64E10) XP® Rabbit mAb (green). Actin filament were labeled with DY-554 phalloidin. Blue pseudocolor = DRAQ5 #4084 (fluorescent DNA dye).
PINK1
PTEN-induced kinase 1 (PINK1) is a mitochondrial serine/threonine protein kinase that protects cells from stress-induced mitochondrial dysfunction. It accumulates on the outer membrane of severely damaged mitochondria and recruits PARKIN to induce degradation through autophagy. PINK1 mutations are linked with autosomal recessive early onset Parkinson’s disease.
Products
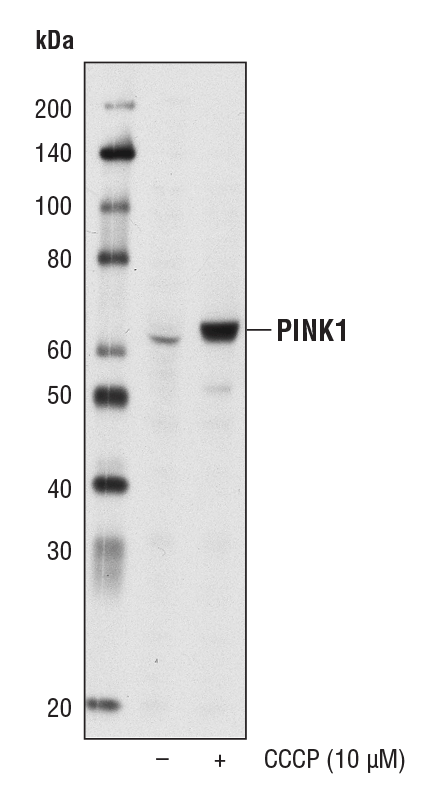
PINK1 (D8G3) Rabbit mAb #6946 – W, IP
PINK1 (D8G3) Rabbit mAb #6946: Western blot analysis of extracts from HeLa cells, untreated (-) or treated with CCCP (10 μM, 24 hr; +), using PINK1 (D8G3) Rabbit mAb.
SQSTM1/p62
Sequestosome 1 (SQSTM1, p62) is an autophagosome cargo protein that binds to protein aggregates to target them for selective autophagy. SQSTM1/p62 mutations lead to an increase in intracellular aggregation of α-synuclein, Huntingtin, Tau protein, and beta-amyloid to drive progression of Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease, respectively.
Products
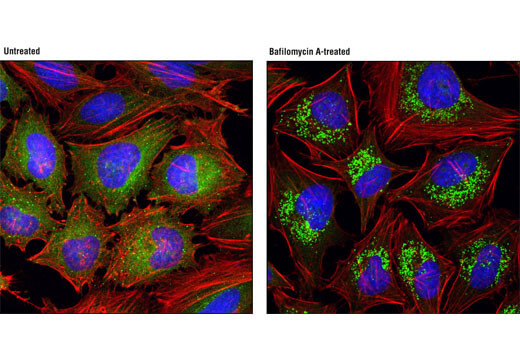
SQSTM1/p62 (D10E10) Rabbit mAb (IF Preferred) #7695 – IP, IF-IC
SQSTM1/p62 (D10E10) Rabbit mAb (IF Preferred) #7695: Confocal immunofluorescent analysis of HeLa cells, untreated (left) or treated with bafilomycin A (100 nM, 18 hr; right), using SQSTM1/p62 (D10E10) Rabbit mAb (IF Preferred) (green). Actin filaments were labeled with DY-554 phalloidin. Blue pseudocolor = DRAQ5 #4084 (fluorescent DNA dye).
LC3A/B
LC3A/B plays a critical role in autophagosome biogenesis and maturation and also functions as an adaptor protein to selectively recruit cargo to the autophagosome. An increase in LC3-positive microglia have been observed in tissues in Alzheimer’s disease patients with TREM2 mutations, suggesting that disruptions in TREM2-dependent autophagy can contribute to Alzheimer’s disease etiology.
Products
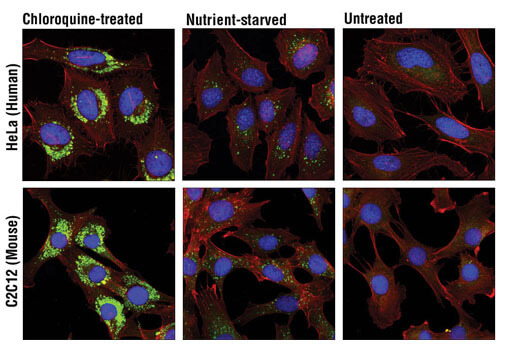
LC3A/B (D3U4C) XP® Rabbit mAb #12741 – W, IHC-P, IF-IC, F
LC3A/B (D3U4C) XP® Rabbit mAb #12741: Confocal immunofluorescent analysis of HeLa (upper) and C2C12 (lower) cells, chloroquine-treated (50 μM, overnight; left), nutrient-starved with EBSS (3 hr, middle) or untreated (right) using LC3A/B (D3U4C) XP® Rabbit mAb (green) and β-Actin (13E5) Rabbit mAb (Alexa Fluor® 555 Conjugate) #8046 (red). Blue pseudocolor= DRAQ5 #4084 (fluorescent DNA dye).
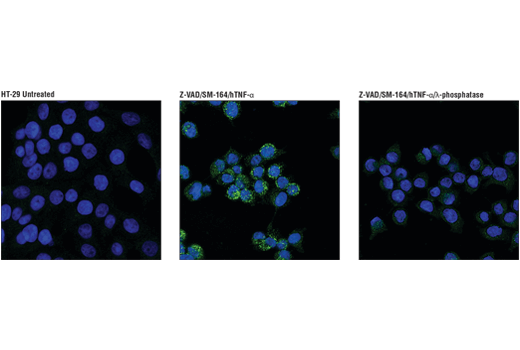
Phospho-RIP3 (Ser227)
Human Phospho-RIP3 (Ser227) phosphorylates MLKL1 to trigger TNF-induced necroptosis. This form of programmed cell death has been reported in multiple sclerosis and amyotrophic lateral sclerosis.
Products
Phospho-RIP3 (Ser227) (D6W2T) Rabbit mAb #93654 – W, IF-IC
Phospho-RIP3 (Ser227) (D6W2T) Rabbit mAb #93654: Confocal immunofluorescent analysis of HT-29 cells, untreated (left), pre-treated with Z-VAD (20 μM, 30 min) followed by treatment with SM-164 (100 nM) and Human Tumor Necrosis Factor-α (hTNF-α) #8902 (20 ng/mL, 6 hr; center), or pre-treated with Z-VAD followed by treatment with SM-164 and hTNF-α and post-processed with λ-phosphatase (right), using Phospho-RIPK3 (Ser227) (D6W2T) Rabbit mAb (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054 (red). Blue pseudocolor = DRAQ5 #4084 (fluorescent DNA dye).